
This video sponsored by Medidata is part of a series examining the role of data analysis in the clinical trials industry

This video sponsored by Medidata is part of a series examining the role of data analysis in the clinical trials industry

Transparency Life Sciences, LLC, and Dr. Matthew Galsky, Associate Professor of Medicine, Division of Hematology/Oncology at the Icahn School of Medicine at Mount Sinai, have collaborated to design and conduct a pilot trial assessing metformin as a potential treatment for prostate cancer.

BBK Worldwide announced the latest version its Study eBinder

Increasingly, biopharma companies are forming relationships with patient advocacy groups.

Study Examines the Power of Biopharma and Patient Advocacy Partnerships in Drug Development

Customized Apple iPad Air Enhances Doctor-Patient Interactions in Clinical Trials

The Association of Clinical Research Professionals is investing $4 million over the next 4 years developing a new state-of-the-art proprietary training curriculum to support the professional growth and development of clinical research professionals worldwide.

The Association of Clinical Research Professionals (ACRP) announced that over the next 4 years they'll be investing $4 million into the development of a new proprietary training curriculum designed for clinical research professionals.

This video sponsored by Medidata is part of a series examining the role of data analysis in the clinical trials industry

PharmaPros Corporation, a provider of integrated technology delivery and services for clinical trials, announced today that the company has changed its name to eClinical Insights, Inc.

This webcast is free and the expert speakers from Price Waterhouse Coopers, Industry Standard Research and SAS will be discussing their own research and experiences on this topic.

Focus on data generation that supports decision making by both regulators and health-technology assessment bodies

New offering improves the quality and predictability of clinical development while reducing overall trial costs by up to 25 percent
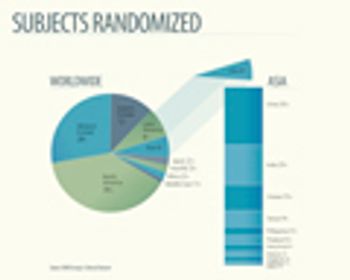

The Berlin-based clinical contract research organizations, ICRC-Weyer and Allied Clinical Management, have announced that they have entered into a strategic alliance, as part of the newly founded MEDIS Research Group
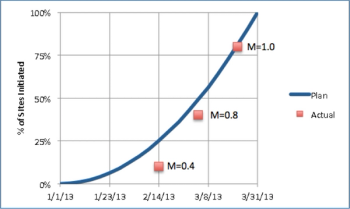
We'll start our monthly metrics blog with a basic but crucial MCC Clinical Trial Performance metric: on-time site initiation.

Synexus has formed a partnership with Northumbria Healthcare NHS Foundation Trust to establish a clinical research facility at Hexham General Hospital, in North East England.

endpoint, a provider of Integrated Voice Response (IVR) and Integrated Web Response (IWR) systems, announced today its expansion into Europe with a European HQ office expected to open in early 2014.

Scimega Research, a Canadian-based specialty oncology clinical research organization, is pleased to announce that it has been selected by The Quebec - Clinical Research Organization in Cancer (Q-CROC) to successfully oversee the conduct of three of pivotal studies to identify predictive biomarkers in drug-resistant tumors.

ICONIK Labs allows visualisation, analysis and reporting on laboratory data in real time

The partnership is based on Oncodesign's Nanocyclix(R) technology platform for next generation kinase inhibitors and UCB?s expertise in neurology

Acquisition Boosts PRA?s Global Phase I Presence

This new country approval is the beginning of a big expansion of CEL-SCI?s Phase III clinical trial to about 20 countries.

Genetic Alliance & PhRMA Announce Pilot Initiative to Advance Patient-Focused Drug Development

As the pharmaceutical industry continues to face increasing cost of drug development, sample banking for future clinical research provides the pharmaceutical industry with new opportunities to obtain biological sample collections that will allow it to investigate safety and efficacy in future clinical research and answer regulatory authority questions related to safety and efficacy at the time of registration.

Sample banking for future clinical research provides the pharmaceutical industry with new opportunities to obtain biological sample collections.

The European Medicines Agency (EMA) and the United States Food and Drug AdministrationExternal link icon (US FDA) have published a second joint question-and-answer document that provides guidance on the quality-by-design concept.

Wingspan announces that Actelion Pharmaceuticals, a top 20 biopharmaceutical company, has selected Wingspan eTMF as its official electronic trial master file.

ACT 2014 e-media kit

Editorial Director Lisa Henderson talks with N