
While lack of commercial viability is the leading cause of Phase I failures for new drug candidates, efficacy issues dominate as the reason for Phase II failures, according to a new analysis from the Tufts Center for the Study of Drug Development.

While lack of commercial viability is the leading cause of Phase I failures for new drug candidates, efficacy issues dominate as the reason for Phase II failures, according to a new analysis from the Tufts Center for the Study of Drug Development.

Intralinks? Holdings, Inc. (NYSE: IL), a leading, global SaaS provider of inter-enterprise content management and collaboration solutions, and IRB Services? (Institutional Review Board Services), a leading, global provider of research ethics review and related services for the clinical research and behavioral science industries, striving for excellence in human research participant protection, today announced that the IRB Services team has selected Intralinks VIA? to support all of its secure beyond the firewall collaboration needs.

To fulfill the need for global biometrics solutions, CROS NT SRL today announced the acquisition of Stat?Tech Services, LLC ? a Contract Research Organization (CRO) with extensive experience in the medical devices sector.

Almac today announced they are now offering a Tumor Profiling service running Illumina?s next-generation sequencing (NGS) TruSight Tumor? panel as part of their biomarker discovery, development and delivery solutions.

Computer simulation will be able to mimic drug disposition in the lungs at different stages of tuberculosis infection

CTI Clinical Trial and Consulting Services (CTI) announces that it has opened an office in Milan, Italy. The office is opened through its newly created wholly-owned subsidiary, CTI Clinical Trial and Consulting Services Italy, S.R.L.

The process of bringing a new molecular entity or device to market has historically been long, costly and both paper and people intensive.

Externally validated program sets global standard for measuring competence of clinical monitors

The International Academy of Clinical Research (IAoCR) welcomes the news today from the Nursing and Midwifery Council that it is introducing three-yearly checks for nurses and midwifes from the end of 2015 to ensure they are fit to practice.

Theorem Clinical Research has announced the addition of Ximedica, a medical product development firm, to its roster of strategic alliances.

CenterWatch, a leading publisher of clinical trials information for patients and professionals, announced today that it has launched a revamped version of its award-winning Clinical Trials Listing Service (CTLS) to better serve the public and patient communities.

Combined technology solution evaluates study data within the EDC system against contract criteria and generates financial transactions and all related accounting information

Kenneth Kirby, the president of TransDermal Delivery Solutions Corp., sees his company?s transdermal drug delivery system as transforming the way we think about medication.

Alliance increases focus on bringing more clinical research to Indiana and provides quicker access to patients for complex Phase I clinical trials
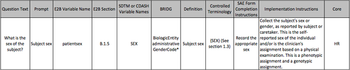
Clinical research is complicated, and it?s not just the science. Take CDASH, CDISC and E2B for example. Aren?t acronyms awesome?

ACT December 2012 BPA Statement

Parexel's President & COO Mark Goldberg sat down with Applied Clinical Trials for a quick discussion on the trials market in Asia Pacific.

Industry news focusing on the people and organizations who work in the clinical trials profession.


There are signs of trouble in the latest announcement of user fees for 2014 authorized by the Prescription Drug User Fee Act (PDUFA).

We can simultaneously establish a database that identifies signals of risk for specific product classes, and also demonstrate products that might in fact reduce risk of suicidal ideation and behavior.

Industry news focusing on the people and organizations who work in the clinical trials profession.
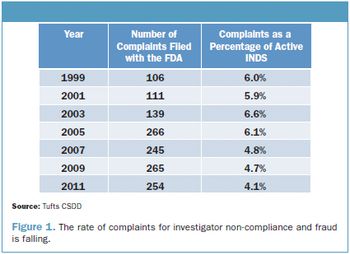
Tufts CSDD

European Union enlargement with Croatia will be discussed in-depth during a regulatory town hall meeting at the Drug Information Association's 26th Annual EuroMeeting.

Trial Design: Streamline and Improve Start-Up Information Technology: Electronic Data Capture Subject Recruitment: Pharmacists and Recruitment Also in this issue: European Data Transparency Vendor Management Suicidal Ideation and Behavior

This e-book provides articles on triggered monitoring, targeted source document verification, and insights from CROs and Pharma executives have tackled, and continue to strategize, on their RBM plans.

A triangle ? an inverted black triangle ? will be seen on the pack leaflets and summary of product characteristics (SPC) of certain medicines in Europe from next week.
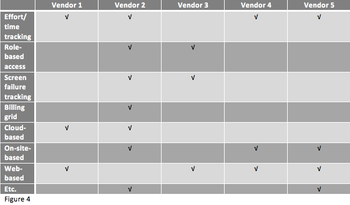
The excessive cost of clinical trials mixed with the inefficiency of clinical trial management is a widespread problem among research institutions.

With the release of FDA's Guidance on Risk-Based Monitoring (RBM), many clinical operations personnel have been asking how they could implement RBM strategies.