
No matter how long you?ve been in the clinical trials business, the mantra is always the same: not enough investigators, not enough patients, enrollment falling behind, need for rescue sites, study budget overspend.

No matter how long you?ve been in the clinical trials business, the mantra is always the same: not enough investigators, not enough patients, enrollment falling behind, need for rescue sites, study budget overspend.
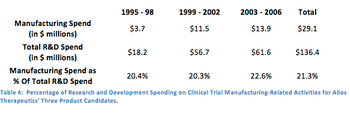
The clinical trial industry often believes that the costs of supplying drugs to clinical trials are a less significant contributor to the overall costs of running clinical trials.


Everyone is looking to rev up the biopharmaceutical development pipeline, and the FDA is working hard to do its part.

The implementation of best practices for clinical study and development conduct can streamline administrative burdens for investigator staff as well as study teams, and hopefully yield reduced costs in conducting global clinical development.

Simulator helps sponsors to assess arrhythmia risk before a candidate enters clinical trials; determine safe drug doses for specific patient populations.

Are clinical trial data shared sufficiently today?

Craig Wozniak, head Americas region, clinical operations at GlaxoSmithKline, discusses TransCelerate and it's impact on the clinical trials industry and risk-based monitoring.


Lori Convy, assistant director clinical research monitoring, Sanofi discusses risk-based monitoring

Register now for the Risk-Based Monitoring Conference! October 24-25, 2013 | Philadelphia, Pennsylvania More on the Risk-Based Monitoring Conference



Craig Wozniak, head Americas region, clinical operations at GlaxoSmithKline, discusses TransCelerate and it's impact on the clinical trials industry and risk-based monitoring

Industry news focusing on the people and organizations who work in the clinical trials profession.

Craig Wozniak, head Americas region, clinical operations at GlaxoSmithKline, discusses TransCelerate and it's impact on the clinical trials industry and risk-based monitoring.

Lori Convy, assistant director clinical research monitoring, Sanofi discusses risk-based monitoring

Jules Mitchel of Target Health discusses risk-based monitoring trends in the clinical trials industry

Crown Bioscience, Inc., a leading global drug discovery and development service company, today announces that it has acquired the shares of Preclinical Oncology Services Limited (PRECOS), a leading pre-clinical research and development service provider with a specific focus on oncology.

Jules Mitchel of Target Health discusses risk-based monitoring trends in the clinical trials industry

Craig Wozniak, Head Americas Region, Clinical Operations at GlaxoSmithKline, discusses TransCelerate and it's impact on the clinical trials industry and risk-based monitoring

A recent Twitter follower asked in regard to RBM technology, "Is there any experience w/regulators utilizing tool data as a roadmap to ID trouble sites during inspections?"

CRF Health, the leading electronic Clinical Outcome Assessment (eCOA) solutions provider announced several innovative enhancements to their TrialMax eCOA solutions.

Collaboration greatly reduces cost and administrative burden in clinical research.

Accessing and engaging patients is a very difficult task in today’s environment, as there is a bombardment of numerous media vehicles, including digital media, with varying intensities that penetrate patients’ senses, emotions, and thoughts.
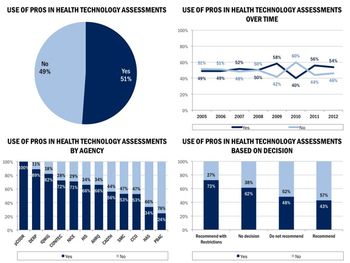
Physicians are increasingly advocating a patient-centered approach to practicing medicine, and both regulatory agencies and manufacturers also seem to agree that patient reported outcomes (PROs) are valuable.

Clinical Research Organization Utilizes M-Files ECM for FDA 21 CFR Part 11 Compliance; Integrates M-Files into SharePoint and Dynamics SL

Offices in Osaka and Shinagawa in Tokyo provide strong clinical development hub for customers in world’s second largest pharmaceutical market
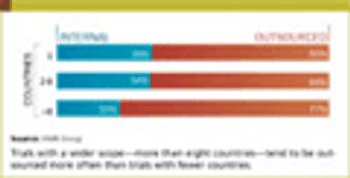

On January 23, 2013, the Department of Health and Humans Services (HHS) published the Omnibus Final Rule (Final Rule) as a modification to the "HIPAA Privacy, Security, Enforcement, and Breach Notification Rules under the HITECH Act and the Genetic Information Nondiscrimination Act of 2008."