News


Last year, I attended the Sponsor/CRO Business Process Integration conference at this same time in New Brunswick, and it was one of my favorites. This year, it's in Raleigh NC.

Acquisition provides growth opportunities and increases synergies within the ProScan Family of Companies

Clinical Ink, a provider of eSource solutions has announced a $4.3 million investment from FCA Venture Partners.

Andy Townshend, vice president alliance development at INC Research discusses functional service provider relationships with Applied Clinical Trials



Novella Clinical, a Quintiles company, is a full service clinical research organization (CRO) and today announced it has been selected by Scioderm to manage a Phase IIb trial for the treatment of Epidermolysis Bullosa (EB),a rare genetic connective tissue condition.

Europe moved one step closer to a passport for physicians in early October.

Pie Medical Imaging BV (PMI) announced today that they have signed an agreement with the Cardiovascular Research Foundation (CRF) to begin a close collaboration.

We've been working with our friends at Medidata on a series of online edicational tools on the topic of integrating IRT and EDC.

Latest ICONIK service enables more informed, data driven decisions regarding patient treatment and safety

The Clinical Conductor Site CTMS development team has just released the third major update of the year. Driven by customer input and a deep understanding of market needs, Clinical Conductor Site has developed a multitude of new features, while enhancing many existing tools.

With so many sponsors and CROs focused on finding the right technology solution for implementing risk based monitoring, a key element often gets overlooked--the risk assessment.

Due to increasing costs and lacking productivity of the existing clinical trial operational model, there has been a lot of awareness around ?Future Clinical Trials,? and some sponsors are seeming to transform pharmacies into study sites.

How Punit Dhillon, CEO, OncoSec Medical, hopes to leverage online communities to design better oncology trials.

Clinical Conductor CTMS?s parent company, Bio-Optronics, Inc., and the Society of Clinical Research Sites (SCRS) have collaborated to expand and advance the knowledge of clinical trial site management challenges and opportunities.

Officials from the Food and Drug Administration and the National Institutes of Health were scheduled to explain developments in clinical trial registration and transparency at the Drug Information Association?s conference on Clinical Trial Disclosure in Bethesda, Md. this week.
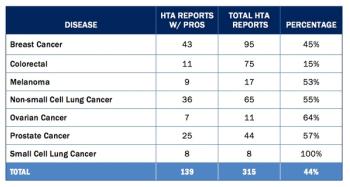
In a recent piece published in the New England Journal of Medicine, oncologist Dr. Ethan Basch talks about his perspective that patient-reported outcomes (PROs) are not widely used in the development process for cancer drugs because they are not often measured in clinical trials.

An analysis of pharmaceutical talent trends has revealed that the industry must address the looming skills shortages or risk losing the best candidates to other sectors.

To provide better support for the pharmaceutical, biotechnology, and medical device industries, Viroclinics has started a specialized group for clinical trial operations.

Sparta Systems and Veeva Systems announced plans to deliver a complete, fully integrated quality solution

CROMSOURCE, an international full service contract research organization (CRO), today announced the official opening of new office premises in Paris, France.


Industry Standard Research

Industry news focusing on the people and organizations who work in the clinical trials profession.

The European Medicines Agency has revealed more information about its new organizational structure.

ACT June 2013 BPA

Is it time to realize the extraordinary promise and vision of personalized medicine?