
If you are in clinical research, you may read a headline such as "Thomson Reuters Previews Game-Changing New Solutions for Insights into Clinical Trials" with passing curiosity... along the lines of "what do they have to do with clinical trials?"

If you are in clinical research, you may read a headline such as "Thomson Reuters Previews Game-Changing New Solutions for Insights into Clinical Trials" with passing curiosity... along the lines of "what do they have to do with clinical trials?"

Cloud computing is changing enterprise across all industries, and the recent success of Veeva's IPO is further evidence that the life science industry is no exception.

Given FDA's new guidance on risk-based monitoring (RBM), there has been a lot of interest on identifying critical data points, drafting RBM plans, and executing RBM.

Policies to widen access to patient level data from clinical trials are gaining traction, despite strong opposition from research sponsors that such initiatives will undermine patient privacy and incentives for new drug development.

The European Forum for Good Clinical Practice has a vital role to play in bringing together all stakeholders to work out how to discharge their responsibilities in the framework of the clinical trial protocol for the protection of each consenting participant

Industry news focusing on the people and organizations who work in the clinical trials profession.

Regulatory: Successful Phase III Trial Monitoring Trial Design: Multi-regional Trials Data Evaluation Also in this issue: Compromise in Europe's Rule Debate True Potential of Adaptive Trial Designs Bring Your Own Device

Venn Life Sciences, a growing CRO announces that it has signed a conditional agreement for the acquisition of the trade and certain business assets and liabilities of CRM Clinical Trials GmbH, a German based CRO, for a total consideration of 0.6m Euro.

April might be - as T S Eliot suggested - the cruellest month, but for pharmaceuticals in Europe, this October is certainly a contender to win an award as the busiest month.

Clinovo released a new version of ClinCapture, its advanced open source Electronic Data Capture (EDC) system.
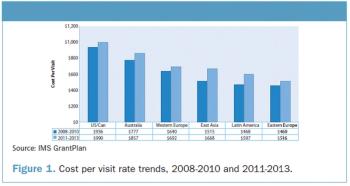
Today's pharmaceutical industry faces many challenges as drug development costs continue to climb, pricing pressures mount, and new global markets gain critical importance.

A conversation with Mary Flack, CEO of Elevated Clinical Trials, on an innovative paperless approach to clinical trial record keeping and monitoring

Over the past several years, we have been seeing an influx of significant changes in the clinical trials industry ranging from changes in the way we approach clinical trials, and massive growth in new technologies.

Over the past few months the majority of the news coming out of the pharmaceutical industry has been a refrain of slashing R&D spending as a result of increasingly competitive drug development.

That's one of our key aphorisms here at the Metrics Champion Consortium (MCC). We passionately believe that measurement can effect change, and we?ve spent the last eight years working with our members to define the best metrics for tracking performance and improvement in the industry.

Synexus has opened a regional office for Eastern Europe in Wroclaw, Poland. The investment reflects Synexus? continued commitment to the region and has been created to service the important role that clients see for Eastern Europe in terms of global clinical trials.

Validation - a word we use a lot in this industry. For Triumph Consultancy Services, it is usually a term which we associate with systems development, but on this occasion I'm excited to say I'm talking about validation of our new business division and its ideas.

Cloud platform supports CRO?s global client base and long-term paperless TMF strategy

Advancement allows for clinical research data in agency-required format and the ability to conduct full-service projects in house.

Study may provide further insight on treatment options for BRCA1 and BRCA2 positive breast cancer patients with metastatic disease

Triumph are proud to release the following position paper: Designing Risk Detection Metrics for Risk-Based Monitoring

Clinverse, Inc., provider of an end-to-end, cloud-based financial lifecycle system for automating global clinical payments, announced that Naurex Inc. has implemented ClinPay to automate investigator payments for a current Phase II trial.

Almost daily, we learn of stunning new advances in machine-to-machine (M2M) technologies in healthcare and life sciences, where sensors collect vast amounts of data that yield immediate and long-term insight, and that carry the promise of improved outcomes and quality of life.

Certara customers will now be able to view curated life sciences content from Thomson Reuters Cortellis alongside their proprietary scientific data

Banook Central Imaging selected by Servier to provide innovative image analysis services based on a combination of RECIST, IrRC and TGR criteria

Radiant Research, Inc., a leading provider of clinical and consumer product testing, has expanded its consumer division.

DrugDev, an interactive network and data sharing platform for over 80,000 clinical trial doctors in 93 countries, announced today its acquisition of CFS Clinical (CFS), the leading specialty provider focused on the business and financial management activities for clinical trials.

Next Generation eTMF System Overtakes Existing Competitor?s Offerings: Innovative Cube Technology Transforms Regulatory and Data Management Organizations to Provide a Competitive Edge.

Applied Clinical Trials Editorial Director Lisa Henderson on the key takeaways from the 2012 Integrating Sponsor and CRO System conference, originally released October 2012