
Last September, inVentiv Clinical Trial Recruitment Solutions announced its strategic partnership with ViS Research to address the challenges of clinical trial site evaluation

Last September, inVentiv Clinical Trial Recruitment Solutions announced its strategic partnership with ViS Research to address the challenges of clinical trial site evaluation

ACM Global Central Laboratory today announced it has acquired Phoenix Pharma Central Services Pte. Ltd

Traditionally, clinical trial management systems have been designed as stand-alone applications for either research sites or for organizations

ACM Global Central Lab announced it has acquired Phoenix Pharma Central Services

To believe the European Parliament, the battle to create new clinical trials rules for the European Union is over.

FDA will present a webinar on the final guidance for industry Electronic Source Data in Clinical Investigations
As the importance of translational research continues to grow, research institutions are exploring other options to remain competitive players in the academic clinical research arena.

There is a similar trend in healthcare and clinical research: finding ways to better connect patients with the medical services and therapies they need at exactly the right time.

After two years in budget limbo, Congress finally enacted federal spending legislation for fiscal year 2014 last week, just before the latest funding extension ran out.

Clinverse, Inc. announced today that it has formed a strategic partnership with Datatrak

AG Mednet has announced the availability of new capabilities to enable sponsors, CROs and core laboratories to take delivery of electronic trial data

Clinverse, Inc. has established a strategic partnership with Datatrak.

AG Mednet announced the availability of new capabilities to enable sponsors, CROs and core laboratories to take delivery of electronic trial data

The first conference I?ll attend this year is in two weeks in Philadelphia, on Data Disclosure and Transparency.

Many biopharmaceutical enterprises and CROs are trying to establish solid risk assessment techniques and infrastructures to enhance clinical trial data quality, strategy, and reduce monitoring costs
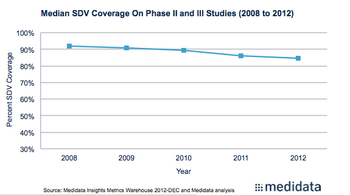
With all of the buzz and excitement around risk-based monitoring, one might expect that the broad adoption of this emerging clinical research paradigm is well under way.
New SMART feature provides CRAs with efficient control of modification requests, eliminates delays and regulatory concerns over site staff activation and deactivation

Exco InTouch announced the successful completion of an independent audit

Exco InTouch announced that it has successfully undergone and completed an independent audit to assess compliance utilizing the National Institute of Standards and Technology guidelines for Health Insurance Portability and Accountability Act security.

Managing billing compliance is one of the most widely discussed topics in the clinical research community.

Quintiles was named to the CSO40, which is for security professionals in a broad range of disciplines.

Quintiles' Trusted Cloud Services Model Recognized in CSO40

BioClinica and CCBR-SYNARC have signed an agreement to merge their companies

Merger to Create Leading Provider of Specialty Outsourced Clinical Services

The Impact of Outsourcing Partnerships on Clinical Trial Optimization
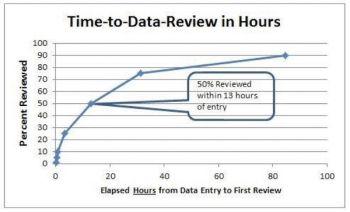
Results from a Multicenter Clinical Trial Using a Quality by Design Methodology, Risk-Based Monitoring and Real-Time Direct Data Entry
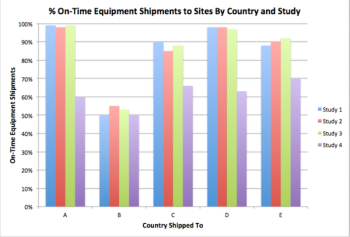
MCC has 100 different metrics relating to clinical trials, from timeliness and cycle time metrics to quality, efficiency, and cost metrics.

As expected, the Food and Drug Administration approved only 27 new molecular entities (NMEs) in 2013.

Significant Enhancements Demonstrate MedNet's Commitment to Efficiency and Ease-of-Use

EMA webminars on eSignatures