
In order to manage critical clinical trial information, sponsors and CROs typically implement a strict data management structure...

In order to manage critical clinical trial information, sponsors and CROs typically implement a strict data management structure...
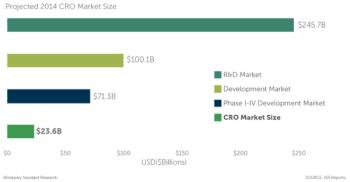
Those of us who have spent considerable time working in the CRO industry have our own version of a "constant" and that is constantly asking the same question: What is the size of the CRO market?

Oracle will integrate Nuance Communications' cloud-based medical speech recognition technology


The European Union has a new research program, but will it help, or hinder, clinical trials?

The decline in important new medicines reaching market in 2013 has produced multiple proposals for making clinical trials more effective and efficient.


Devoting time to work on "correctable weaknesses" of research design will pay off in the long run.

TransCelerate BioPharma has provided the first update to its position paper on risk-based site monitoring

Infinata announced its SiteSurvey

At this year's annual SCOPE in Miami, data management and information services provider, Infinata, Inc., will unveil SiteSurvey

Six leading organizations selected the OnCore Enterprise Research system in 2013


Forte Research Systems announced that six leading clinical research institutions selected the company?s enterprise research system last year.

Camargo Pharmaceutical Services recently completed an extensive IT system update

DS Healthcare Group, Inc announced it has appointed Aptiv Solutions as CRO for its proprietary topical prescription treatment.

Camargo Pharmaceutical Services recently completed an extensive IT system update

The Belgian Association of Clinical Research Professionals has presented its European outstanding leadership award

Generating a complete schedule for a hospital department or specialty practice can be a highly complex process.

DS Healthcare Group, Inc announced it has appointed Aptiv Solutions as its CRO for its proprietary topical prescription treatment.

The drug industry and drug regulatory authorities have been among the most conspicuous targets of this intense monitoring

ClinPay Solution Will Manage Complete Financial Lifecycle for Global Trial

ClinPay Solution Will Manage Complete Financial Lifecycle for Global Trial
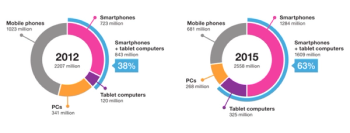
It is tempting to imagine the use of the patient's own mobile computing platform for collection of patient reported outcomes.

Novotech has expanded its clinical services with a new office in Johannesburg, South Africa

BBK Worldwide is suggesting that companies consider global streaming radio as a way to reach potential clinical trial participants.

The Drug Information Association has posted 13 new video clips

Collaboration is the way clinical research is moving.

The complex legal, regulatory, financial, and organizational requirements are not widely known...

With increasing challenges surrounding clinical trial subject enrollment and engagement, the landscape of clinical trials continues to morph.