
Employee turnover remains high in the US and is over 30 percent in some countries.

CRO Pay Incentives
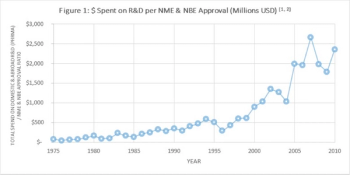
Many of us concur that the biopharmaceutical industry is facing a crisis, and that the R&D process needs to transform entirely in order to address skyrocketing costs and minimal effectiveness.

CRO Employee Turnover

Employees at clinical research organizations (CROs) receive lower annual and long term incentives than their counterparts in other industries.

Drug companies can get more out of their CRO partners by treating them less like avatars and more like equals, according to conference-goers at a CBI event in The Triangle.

Our most read stories of 2013.

The disengagement of major drug firms is described as "a type of market failure" in a briefing paper published last month by the UK think-tank Chatham House.

EFPIA welcomes the agreement on the EU Clinical Trials Regulation concluded by the legislators today.

Arsenal Capital Partners announced today the acquisition of Certara,

Many Israeli pharmaceutical executives recently learned that the 505(b)(2) development process is an important tool that can significantly reduce costs and shorten the timeline for approval of new drugs, adding substantially to ROI.

The European Medicines Agency has now reviewed all comments received on its draft policy on publication and access to clinical trial data.

It will then be standard practice to publish the agendas at the start of each meeting and the minutes after their adoption the following month.

The Association for the Accreditation of Human Research Protection Programs today announced that it has awarded accreditation to five more organizations

SPIRITT Selects iMedNet EDC for its eClinical Flexibility and Ease of Use

Siemens Healthcare Diagnostics Inc. announces that it has entered into a master collaboration agreement with Pfizer Inc.

Siemens Healthcare Diagnostics Inc. has entered into a master collaboration agreement with Pfizer
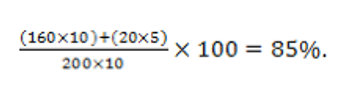
Quality metrics are hard to define and measure, but are crucially important.

Quotient Clinical has been acquired from the Quotient Bioresearch Group by Bridgepoint Development Capital for an undisclosed sum

Quotient Clinical has been acquired from the Quotient Bioresearch Group by Bridgepoint Development Capital.

The final version of the EMA Reflection Paper on Risk Based Quality Management in Clinical Trials is now available.

Three different drugs developed in Finland have received a marketing authorization during the past year, and a positive decision is expected on the application of a fourth marketing authorization.

When serious things go wrong with a trial, such as an adverse event or an incident report is filed, it is easier for project managers to identify a problem that needs to be addressed in a trial.

Three different drugs developed in Finland have received a marketing authorization during the past year, and a positive decision is expected on the application of a fourth marketing authorization.

Cloud-based platform provides real-time data insights for clinical trials

With the average clinical trial using of 5-7 different clinical systems, one of the critical business challenges the industry faces today is turning these siloes of data into operational intelligence

Venn Life Sciences, a CRO providing clinical trial management and resourcing solutions to pharmaceutical, biotechnology and medical device clients, announces the recent acquisition of two European CROs.

Venn Life Sciences, a growing Clinical Research Organization providing clinical trial management and resourcing solutions to pharmaceutical, biotechnology and medical device clients, announces the recent acquisition of two European Clinical Research Organizations.

Innovative uses of social media and other new information technology has become increasingly important in improving clinical research operations and sponsor communications with sites, investigators and vendors.