
In our increasingly online world, it only makes sense that more and more people are turning to the Internet to find and share information about their health and wellness.

In our increasingly online world, it only makes sense that more and more people are turning to the Internet to find and share information about their health and wellness.

Pharmaceutical and medical products companies started to collect data on payments and "items of value" provided to teaching hospitals and physicians, as required by the Sunshine provisions.

CRO/Sponsor: The State of the Union of Sponsor and CRO Relationships Subject Recruitment: Pharmacists Can Raise Patients' Research Literacy Also in this issue: European Policy on Clinical Trials DNA Check at Home Drug Effects

ERT, a global solution provider for patient safety and efficacy endpoint data collection, announced that the Journal of Clinical Psychiatry (JCP) has published a paper demonstrating the effectiveness of the electronic, patient-reported version of the Columbia Suicide Severity Rating Scale - also known as the eC-SSRS - in assessing suicide risk among clinical trial participants.

The Research Triangle is no stranger to the pharmaceutical industry, but newcomer Clintrax Global, Inc. is changing how the industry thinks about clinical trial contract negotiations.
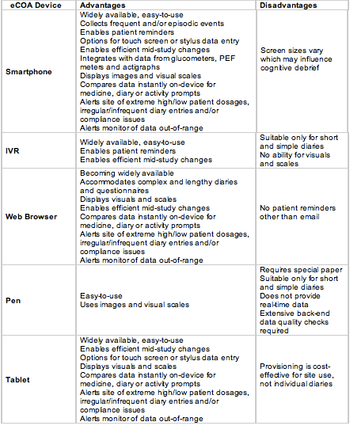
The specialized industry of collecting patient-driven eData is increasing exponentially. Patient-driven eData encompasses all electronic clinical outcome assessments - or eCOAs -- including patient reported (PRO), clinician reported (ClinRO) and observer or caregiver reported outcomes (ObsRO).

Covance Inc. (NYSE: CVD) announced the launch of external laboratory management services , a new offering that provides biopharmaceutical companies with a comprehensive management solution for external laboratory testing associated with clinical trials.

Virtify CTRR Integrates with OnCore Enterprise Research System

In July 2004, FDA Commissioner Lester M. Crawford, FDA Commissioner announced the desire of the FDA to receive data in a standard format, the CDISC SDTM.

Almac announced the launch of SupplyTraQ?, a web-based drug accountability and reconciliation tool that for clinical trial drug supply management and post-trial administration.

Europe's closest thing to an Oscar's ceremony for bioscience will take place in early October.

Undertakes Novel Genomic ?Pre-profiling? Feasibility Study to Examine the Promise of Precision Medicine in Metastatic Colorectal Cancer Patients

In Part 1 of this article, I described a collaboration between Brock Heinz from Spaulding Clinical and Joe Dustin from Medidata, which resulted in a very interesting proof-of-concept.

Before the Orphan Drug Act of 1983 the FDA and lawmakers considered designation and incentives for orphan development without much consideration for competitors in the same orphan space.

With all the discussion in Europe of how to move towards personalized medicine and more flexible authorization procedures, it is the rare disease community that is, in many ways, offering guidance on the route ahead.

FDA recognizes the investment made by sponsors over the past decade to develop the expertise and infrastructure to utilize Clinical Data Interchange Standards Consortium (CDISC)[1] standards for study data. T

Almac?s Clinical Services business unit announced the addition of refrigerated reusable shippers to its portfolio of solutions for the management and global distribution of temperature controlled clinical supplies.

inVentiv Clinical Trial Recruitment Solutions (iCTRS), announced its strategic partnership with ViS Research to address the challenges of clinical trial site evaluation.

C.?The Association for the Accreditation of Human Research Protection Programs Inc. today announced that it has awarded accreditation to four additional organizations, including one more in Korea.
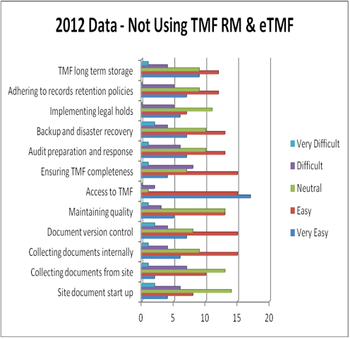
The research and development process continues to be long, complex and costly. Sponsors are challenged with filling pipelines, conducting complex global clinical trials and complying with requirements for additional safety and efficacy data.

A conversation with Marc Buyse, founder of CluePoints on risk based monitoring in clinical trials

A conversation with Marc Buyse, founder of CluePoints on risk based monitoring in clinical trials

In August of 2013, the FDA released a report that analyzed clinical trial subject demographic subgroups for FDA approved medical products, which included factors such as race, age and gender.

A conversation with Marc Buyse, founder of CluePoints on risk based monitoring in clinical trials

An agency reshaped for the future. The European Medicines Agency (EMA) has announced details of its new organizational structure.

The Omnibus Final Rule (Final Rule) entitled "Modifications of the HIPAA Privacy, Security, Enforcement, and Breach Notification Rules under the HITECH Act" became effective on March 26, 2013.

As clinical researchers, it?s natural for us to think of patients first and foremost as research participants (potential or current). But that?s not how patients think of themselves.

The Omnibus Final Rule (Final Rule) entitled "Modifications of the HIPAA Privacy, Security, Enforcement, and Breach Notification Rules under the HITECH Act" became effective on March 26, 2013.

Theorem Clinical Research has announced the formation of a strategic alliance with Charles River Laboratories that will provide clients with an integrated solution from nonclinical testing and analysis through clinical development and registration.