
News






Industry news focusing on the people and organizations who work in the clinical trials profession.

Risk-based monitoring was in Trans-Celerate BioPharma's sights since the pharmaceutical company collaborative was announced in September 2012.

Food and Drug Administration officials are busy implementing its provisions that offer new approaches to clinical trial design and application review.

Treatment networks and network meta-analysis of randomized trials can boost understanding of how much evidence is available for each treatment and treatment comparison.
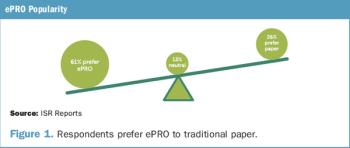
Industry Standard Research

Trial Design: Cystic Fibrosis Clinical Trials Patient Retention Strategies PRO in Analgesia Trials Subject Recruitment: Community-Based Recruitment Also in this issue: Female Trial Subjects Data Transparency Mitigating Risk















This year, CBI will be co-locating their Risk-Based Monitoring in Clinical Studies conference and Clinical Patient Engagement Summit this October 24-25, 2013 in Philadelphia.



In the podcast, Kent Thoelke, Executive Vice Presdient, Scientific & Medical Affairs for PRA, discuss parallel drug development strategies in China, the nuances of the regulatory market there, as well as best practices.
