
News















Negotiating skills can be applied to clinical trial agreements, budgets, and more for effective and fair contracts.


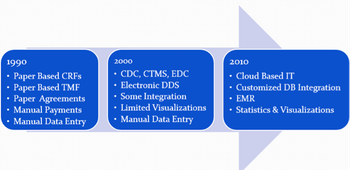
In recent documents and presentations, officials in the OSI in the CDER are highlighting the value of building quality into the design and operation of clinical trials to gain more efficient and effective monitoring and data verification systems.

Industry news focusing on the people and organizations who work in the clinical trials profession.




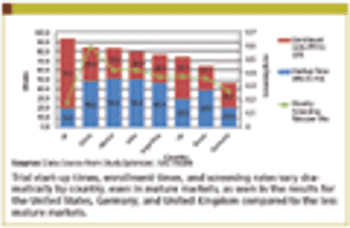
Monitoring patient safety is an integral and critical part of the clinical trial process.

A researcher from a top US institution has delivered strong criticism of Roche's promise to release full clinical study reports as part of its data transparency policy, signaling an escalation of the battle over publication of clinical trial results.

A view on redefining key roles across the evolving clinical development landscape.


CRO/Sponsor: Strategic Partnerships in Trial Outsourcing Trial Design: The Future of Clinical Monitoring Project Management: Negotiating Effective Agreements and Budgets Also in this issue: Trial Debate in European Parliament Redefining Key Roles Biospecimen Storage
