
News






Active evaluation of non-core procedures is an unusual winwin opportunity for sponsors and CROs.
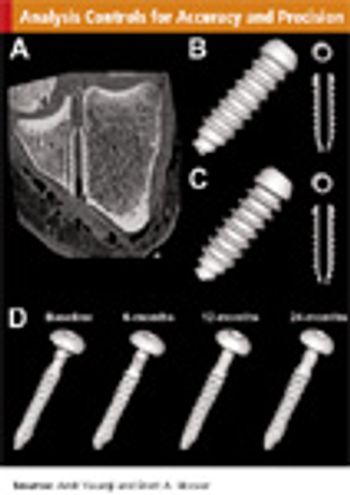
Incorporating imaging analytics can improve the quality of work and minimize unexpected delays.

Industry news focusing on the people and organizations who work in the clinical trials profession.

The long wait is finally over for muchanticipated regulations governing disclosure of pharmaceutical company payments to physicians, and on the rights of patients to protect access to their personal health information (PHI).
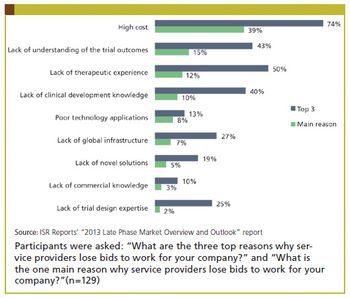
Industry Standard Research
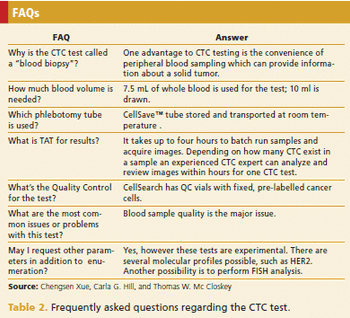
A CTC test may fulfill an unmet need in oncology clinical trials and disease management practices.

Increasingly strict regulatory controls governing clinical research pose a severe threat to the development of new cancer medicines for children, and may jeopardize recent improvements in survival, according to a special edition of Lancet Oncology.

The FDA has released its plan for pre-dementia Alzheimer's trials.


Constant technological advancements continue to alter the compliance battle.

How one eClinical platform can create unparalleled efficiency in the future.



Path to Patient Drug Compliance in Clinical Trials. Podcast slideshow interview with Tony Frankland, Global Head of Sales and Marketing Cenduit






Lisa Henderson interview Gerd Arold or PRA about patient recruitment factors in early phase trials

Lisa Henderson interviews Gerd Arold of PRA about patient enrollment in early phase trials in Eastern Europe



