
News





















Russian regulators have reportedly implemented the much awaited changes to the nation's regulatory process for medical devices, and the revised regulations took effect at the start of January.

To reduce the time from bench to bedside we need to transform competition into cooperation.


While there is no single correct way to develop process compliance controls to meet federal clinical trials billing regulations around Medicare, standardization of the entire billing process is key.

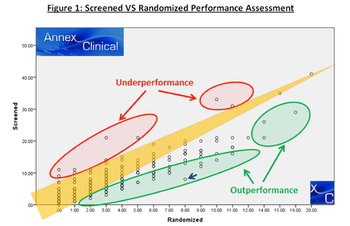
The sponsor of a recent Phase II global study, conducted at more than 50 sites, invested in a centralized digital pathology core lab to ensure a homogenous and representative study population.
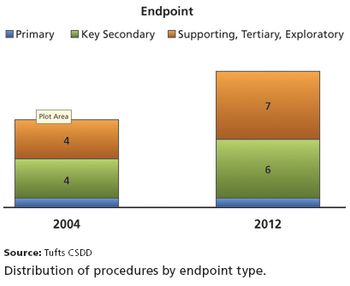

The Food and Drug Administration approved nearly 40 new drugs in 2012, beating most recent totals, and is looking for provisions of the FDA Safety & Innovation Act (FDASIA) to further improve its operations and encourage innovation.

Options have emerged that make DIY EDC technology more accessible to smaller organizations.