
News



















SURVEY : Salary and Career Development Survey Also in this issue : EU Employment, Business and People Update, Investigator Training
Industry Standard Research


Organizations offering courses and classes designed to broaden your clinical trials knowledge base.

Industry news focusing on the people and organizations who work in the clinical trials profession.

There is currently no set of standards for how individuals acquire the ability to perform satisfactorily and demonstrate the required levels of competence.

The primary objectives of GCP are to protect the safety, rights, and welfare of subjects, and to ensure the credibility of trial data and resulting reports.

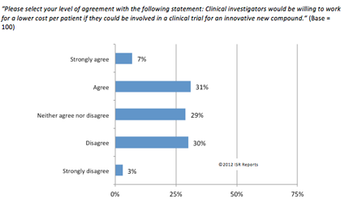
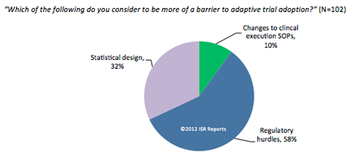
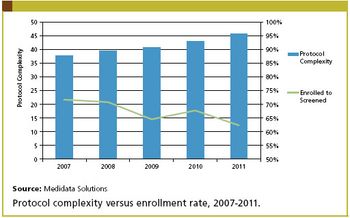
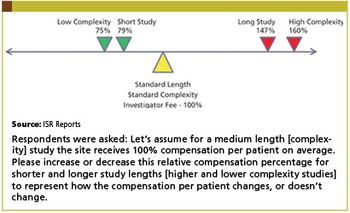
Increasing global competition from generics and a glut of expiring patents have forced many biopharmaceutical and medical device companies to rethink their operational strategies.