Global reach for at-home study visits can accelerate clinical trials, says CEO.

Building a culture of service excellence between sponsor/CROs and investigative sites.

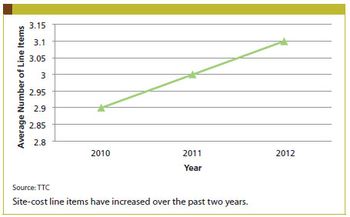
Tufts CSDD

Last October, Outcome Sciences announced its collaboration with the National Football League to update its Injury Surveillance System (ISS), since then, the pilot has been completed during the course of the 2011 NFL season and rolled out to all the teams for 2012.


Current trends in oncology trial terminations as analyzed by Citeline have revealed that sponsors are learning from the past and making gains in this troubled area of drug development.

Why should sponsors and CROs consider using mobile nursing for clinical trials?

ACT 2013 Media Kit









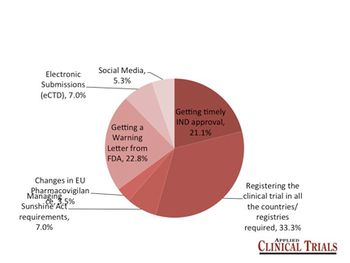
The somewhat-awkwardly named TransCelerate BioPharma immediately got an enthusiastic reception from industry watchers and participants, mainly due to the perception that it was well poised to attack some of the systemic causes of delays and cost overruns that plague clinical trials today.