
SAS - Rethinking Clinical Trials Data Integration



Cetero Research Announces Executive Appointments and New Company Name


Larix A/S Licenses OmniComm Systems' eClinical Solutions

Embedded mobile icon means website can be accessed on the move in just one click

Institutional Review Board Services (IRB Services) has announced it was asked by Trafalgar Ethics Board (TEB) to ensure a smooth transition of operations as it had to close its doors based on business reasons.

Cost inefficiencies in drug development are a major focus of discussion, especially with the many financial impacts affecting the pharmaceutical sponsors.


Industry news focusing on the people and organizations who work in the clinical trials profession.

The percentage of source document verification coverage has been decreasing slightly over the past four years. Therefore, the amount of on-site time required by site monitors should also start decreasing.
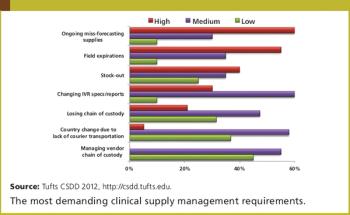
Tufts CSDD
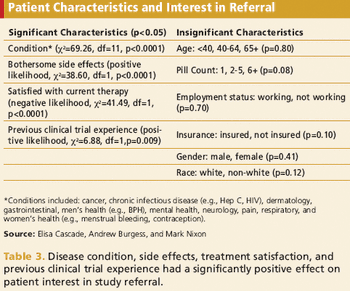
Analysis suggests age, condition, and treatment satisfaction have most significant effect on participation.

In August last year, the FDA came out with its Guidance for Industry Oversight of Clinical Investigations-a Risk-Based Approach to Monitoring. Sponsors and CROs alike are still examining the document and discussing how best to move forward.

Criticisms of the pharmaceutical industry's real motives for undertaking post-marketing studies have been made by a special report in the British Medical Journal.

The main result of the Supreme Court decision upholding the Affordable Care Act (ACA) is that it ends all the rampant speculation and uncertainty about the future shape of the US healthcare system.

Despite their growing role, little benchmark management data, until now, existed on global study monitors.

It's hard enough to conduct clinical trials for experimental medicines, but it can be even more difficult when patients already have access to the medicine outside the research setting.

Regulatory: Developing Biosimilars for a Global Market Subject Recruitment: Patient Interest in Clinical Trial Referral CRO/Sponsor: Functional Service Provider Model Also in this issue: Proposed Rule Changes in Europe Global Study Monitors Benchmarks Emergency Department Trials







Leader in Business Outcomes Provides Program to Help Pharmaceutical Developers and Contract Research Organizations Save Costs, Reduce Cycle Times

Quotient Clinical and Capsugel Announce Collaboration to Accelerate Early Development

Forte Research Systems has Created the Allegro® ePayments System for Research Sites and their Patients.

Strengthened Partnership Facilitates Advancements in Patient Engagement Solutions