
Details on the webinar.










SUBJECT RECRUITMENT : Latino Recruitment - Motivations and Tactics REGULATORY : Medical Device Trials - Regulatory Pathways and Clinical Operations Also in this issue : Clinical Testing Challenges, Ethics in Europe, Enrollment Performance, Phase Labels

Eric Abadie's resignation as Chair of the Committee for Medicinal Products for Human Use of the European Medicines Agency shocked the industry.

One increasingly popular way to help ease the transition into a new role in the clinical trial space is through a mentoring program.

At the DIA EuroMeeting in March a look at Health Technology Assessment (HTA) alongside regulatory authority initiatives was presented.

Form

FDA Draft Guidance "Determining the Extent of Safety Data Collection Needed in Late Stage Premarket and postapproval Clinical Investigations" is reviewed.

ISR Reports


Better selection and tougher site management is not enough to improve enrollment success.

Industry news focusing on the people and organizations who work in the clinical trials profession.

A job seeker's two most used tools are the cover letter and resume.
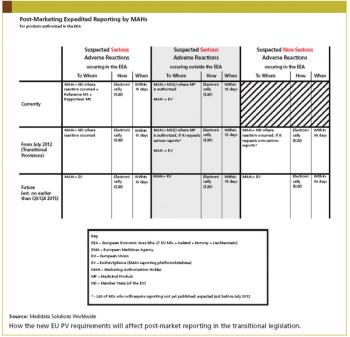
The EMA issued seven draft guidances of the 16 modules for good pharmacovigilance practice in February. There has been confusion and concern among sponsors over how to implement a legislation with no clear guidance from the EMA.
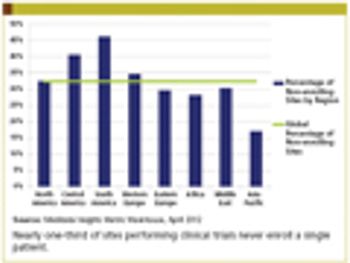
Medidata Solutions






