
News


ZEINCRO, a specialist provider of clinical research services in Greece, Turkey and Cyprus, today announced it is consolidating with Trial Masters Ltd, a contract research organization managing clinical trials in Hungary and Romania.

The Michael J. Fox Foundation for Parkinson's Research launches Fox Trial Finder in the United Kingdom, Ireland and Canada. This first-of-its-kind online platform anonymously connects volunteers with and without Parkinson's disease to clinical trials in critical need of participants. Fox Trial Finder matches volunteers with the trials most likely to need them, increasing the efficiency of the enrollment process and empowering patients to get more involved in the discovery of new treatments.

EU Pharmaceutical Industry Leaders Call for Revision of German Model for Assessment of New Medicines
In a June 8, 2012 meeting in Berlin the leading pharmaceutical companies of Europe, represented in EFPIA, asked the German Government for urgent action to protect patient access to new medicines and ensure that Germany remains a home for pharmaceutical innovation.

In honor of the 15-year anniversary of the founding of the Clinical Data Interchange Standards Consortium (CDISC) and the recently approved CDISC status as a 501(c)(3) charitable foundation, the Annual Report for year 2011 has been released and is available for download on the CDISC website.


Lisa Hendeson talks with Nicki M. Norris, Chief Executive Officer, Clinical Resource Network, about patient centric approaches to patient recruitment and retention









HR Study - White Paper Program - MedNet Medical


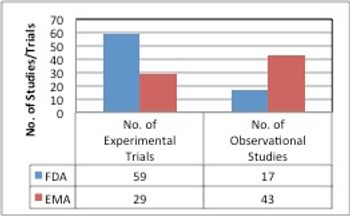
A new study by Premier Research shows that both American and European pharmaceutical companies are facing similar problems in regards to pediatric trial regulations.

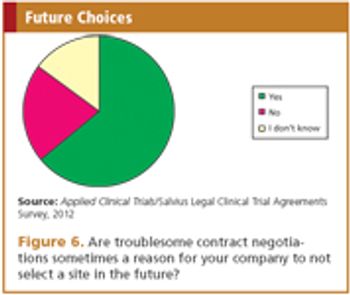
There are opportunities to make the negotiation process more efficient and reduce timelines.

2012 e-media kit - new contact page

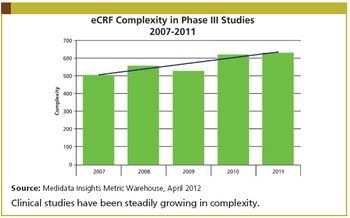
We collect too much data, burdening investigators, monitors, and data managers.

What works when and for whom in the era of comparative effectiveness research.

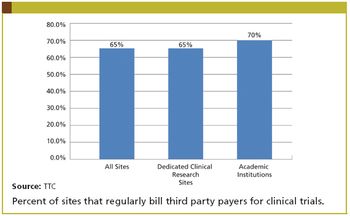
An efficient system can be the difference between a drug being withdrawn or delivered to market.

In clinical trials, the need for globally accessible toll-free and other phone numbers for trial functions is vital.

Industry news focusing on the people and organizations who work in the clinical trials profession.