
News
Advertisement


Advertisement




Advertisement

When the EMA and the FDA both released their views on risk-based approaches to clinical trials in August 2011, a light appeared for sponsors looking for relief in the way they manage resources in clinical trials.













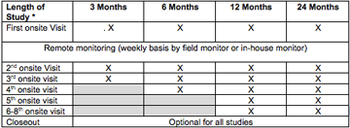
Sponsors, CROs, regulators, sites and opinion leaders have discussed the pros and cons of a risk-based approach to field monitoring for years. The pharmaceutical industry always sought guidance from the FDA, and different approaches for reviewing data collected by sites.
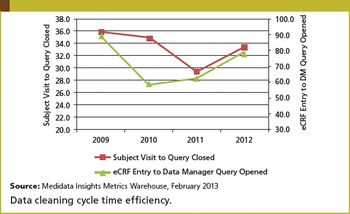

Improving diversity, education, and advancement in studies: a look at the IDEAS initiative.

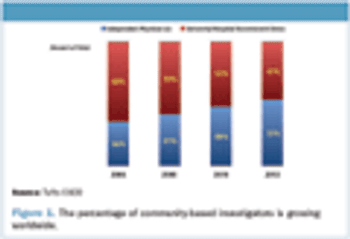
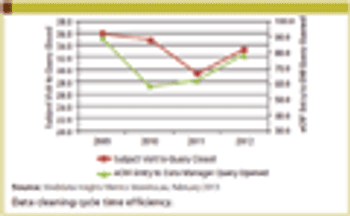
The Avoca Group's "2012 Industry Survey," sponsors and service providers were asked to share their experiences with strategic partnerships.



Advertisement
Advertisement
Trending on Applied Clinical Trials Online
1
SCOPE Summit 2026 Keynote Panel: Is Radical Acceleration in Clinical Research Possible?
2
SCOPE Summit 2026 Panel Discussion: Diversity in Clinical Trials—What’s Working, What’s Next
3
Accelerate Clinical Trials with AI-Enhanced Financial Management
4
SCOPE Summit 2026: Reducing Patient Burden Is the Foundation of Wearable Success in Oncology
5