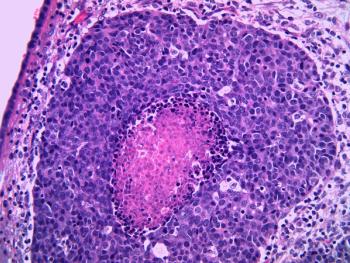
Phase III DIPPER trial shows that adjuvant camrelizumab administered after induction-concurrent chemoradiotherapy significantly improved event-free survival in patients with locoregionally advanced nasopharyngeal carcinoma, along with a favorable safety profile.