
Conference keynote explores the long-term vision for integrating clinical research and care by 2035, including progress driven by digital advancements, artificial intelligence, and evolving regulatory frameworks.

Conference keynote explores the long-term vision for integrating clinical research and care by 2035, including progress driven by digital advancements, artificial intelligence, and evolving regulatory frameworks.

Interactive panel on day 1 of SCOPE Summit 2025 highlighted the need for inclusive narratives, social listening to understand patient experiences, and the role of advocacy groups in opening doors to clinical trials.
Braftovi (encorafenib) plus Erbitux (cetuximab) and mFOLFOX6 (fluorouracil, leucovorin, and oxaliplatin) improved progression-free survival and overall survival compared to chemotherapy in patients with metastatic colorectal cancer with a BRAF V600E mutation.

In this video interview, Michael Andreini, president and CEO of the Multiple Myeloma Research Foundation, highlights how the MMRF accelerates trial timelines for biopharmas by integrating an adaptive platform model.
The multi-center, open-label Phase Ib study, ABC-Pax, will assess the safety and efficacy of the regimen in women with triple negative breast cancer.

The 12-month open-label extension of the PACIFIC trial demonstrated that bexicaserin significantly reduced seizure frequency in patients with developmental and epileptic encephalopathies while maintaining a favorable safety and tolerability profile.

In this video interview, Michael Andreini, president and CEO of the Multiple Myeloma Research Foundation, discusses how MMRF goes beyond safety and efficacy data to help biopharmas research appropriate dosing for multiple myeloma therapies.

Recent survey study of 978 cancer patients and their relatives uncovered reasons behind their willingness, or lack thereof, to participate in clinical research.
The Phase III HYPERION trial was stopped early after strong positive interim results from the ZENITH trial demonstrated the efficacy of Winrevair (sotatercept-csrk) in treating pulmonary arterial hypertension, making it unethical to continue.

In this video interview, Michael Andreini, president and CEO of the Multiple Myeloma Research Foundation, talks unmet need in the multiple myeloma space and how the Horizon trial is addressing complexity in treatment regimens.
Research from the Tufts Center for the Study of Drug Development’s PACT Consortium shows DCTs encourage higher participation across multiple demographic groups.

In this video interview, Michael Andreini, president and CEO of the Multiple Myeloma Research Foundation, highlights the design of Horizon, an adaptative platform trial for the treatment of multiple myeloma.
Abelacimab was found to significantly lower factor XI levels and bleeding events compared to rivaroxaban (Xarelto) in patients with atrial fibrillation at moderate-to-high risk for stroke.

In year two of the trial, Elevidys demonstrated statistically significant and clinically improvements across three key functional outcomes.
The Phase III INAVO120 trial found that a combination of Itovebi (inavolisib) with Ibrance (palbociclib) and Faslodex (fulvestrant) significantly improved overall survival and progression-free survival in patients with PIK3CA-mutated, HR-positive, HER2-negative, endocrine-resistant advanced or metastatic breast cancer.

In this video interview, Michael Andreini, president and CEO of the Multiple Myeloma Research Foundation (MMRF), discusses the greatest challenges with clinical trials in multiple myeloma and how MMRF is aiming to address them.
Being evaluated for the treatment of metastatic colorectal cancer, the combination regimen achieved an objective response rate of 61% compared to 40% for investigator’s choice of chemotherapy.
Phase III CheckMate -8HW trial shows the combination of Opdivo (nivolumab) and Yervoy (ipilimumab) significantly improved progression-free survival and overall response rates in patients with microsatellite-instability–high or mismatch-repair–deficient metastatic colorectal cancer.

In this video interview, Dipanwita Das, CEO & co-founder at Sorcero, highlights trial startup timelines and workforce development as key areas of focus for clinical operations professionals in 2025.

Extended collaboration will further explore the capabilities of Medidata Platform from early-phase trials to post-marketing surveillance.
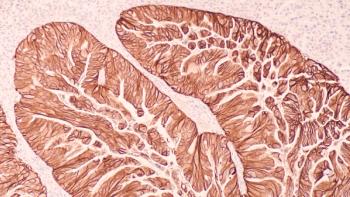
Keytruda (pembrolizumab) plus Lenvima (lenvatinib) and chemotherapy improved progression-free survival but did not achieve statistical significance for overall survival in patients with advanced HER2-negative gastroesophageal adenocarcinoma.

In this video interview, Dipanwita Das, CEO & co-founder at Sorcero, highlights increased data management, DE&I, and regulatory preparedness as key trends for the industry in 2025.

In the Phase I dose escalation portion, which evaluated the combination in participants with small cell lung cancer, the first patient treated attained partial remission.

Phase III Vivacity-MG3 trial shows that nipocalimab, an investigational FcRn blocker, significantly the improves symptoms of generalized myasthenia gravis with a manageable safety profile.

In this video interview, Dipanwita Das, CEO & co-founder at Sorcero, looks back to 2024 and discusses some of the industry’s greatest advancements in using artificial intelligence from the previous year.

End-to-end offering is designed to streamline site activation and optimize visibility in critical research processes.

Use of decentralized approach in a Phase 1 pharmacokinetic trial shows the ability to enable remote data collection and monitoring, which could improve patient access and enhance the efficiency of clinical research.

In this video interview, Dipanwita Das, CEO & co-founder at Sorcero, highlights how artificial intelligence, real-time monitoring, and historical data can aid in optimizing trial design.

How biopharmas are advancing their pharmacovigilance operations.

Investigators find that most exclusion criteria in critical care randomized clinical trials are justifiable, but 60% include at least one poorly justified exclusion, most commonly affecting pregnant or lactating individuals.