
News












SITES : CTMS - Site Productivity Gains Why You Need This 10-Step Public Relations Plan UK Network for Pediatric Research Also in this issue : REMS Addresses Opioids, Adverse Events Assessment in Europe, Academia, NIH Above Repute?, Financial Impact on CROs


Applied Clinical Trials is bringing the conference to you...don't miss it.

Human Research Relies on Accuracy, Honesty, Attention to Details, and Quick and Effective Response of the IRB

Real-life examples of how a clinical trial management system impacted work-life for the better at five sites.

In the IRB World, An Application of Flexibility and Learning to Live with Shades of Grey is Helpful

An International Perspective: Why Were Commercial IRBs Singled Out for Congressional Hearing?

Industry news focusing on the people and organizations who work in the clinical trials profession.

Parexel's Start-up and Accelerated Recruitment Team (START) is applying an integrated approach to data that is to be applied in early phases to alleviate recruitment and study start-up challenges.

Accreditation, Previous Audits, Capacity, and Research Community Support All Factor Into Deciding on an Ethics Board
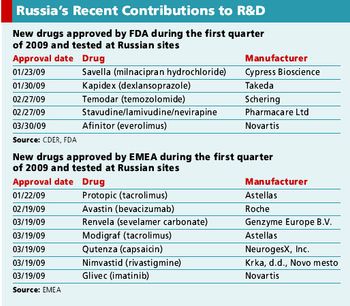
The current state of the clinical trial market in Russia.

After the Coast Hearing, IRBs Should Prepare for More Scrutiny by Focusing on Training, Education, and SOPs

Many Capabilities Need to Be Evaluated by the Sponsor or CRO When it Chooses a Central IRB

The public would be best served if both recognize their own questionable practices.

The latest eclinical software in the clinical trials industry.

All Stakeholders in Human Research Protection Would Benefit from a Cooperative Enterprise That Moves Forward Without Blame

IRBs Can Provide Education and Facilitate Better Relationships to Improve Research Compliance

IRBs Role Today