
News



European Union focuses on adverse drug reaction research.




















EDC : Getting it Right - EDC Rescue Studies INFORMATION TECHNOLOGY : How Health Intelligence Leads to Smarter Research CRO/SPONSOR : The Proactive Advantage in Risk Management Also in this issue : All Eyes on R&D as U.S. Talks Health Care, Fragmented System Stifling EU Research, IT Convergence: Are We There Yet?, Why Virtual Meetings Make Sense

Inside Outsourcing November 2009

ACT June 2009 BPA Statement
The latest eclinical software in the clinical trials industry.
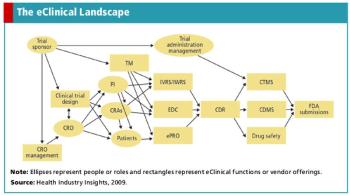
The growing adoption rate of EDC will enable companies to reap the benefits of the entire eClinical suite.

Vital players come together in an attempt to find a cure for a devastating illness predominantly effecting African countries.

A look at the growing market of dietary supplements and the potential for development of these products in the clinical trials arena.