
News
Advertisement


Advertisement

15th Annual Special Resource Issue CDISC Glossaries Professional Societies and Associations Training and Education Comprehensive 2010 Calendar



Lab Network Improves PBMC Sample Quality to Support Clinical Trials - PPD Whitepaper
Advertisement









Remote Data Capture: Acquisition and Analysis - Oracle Whitepaper




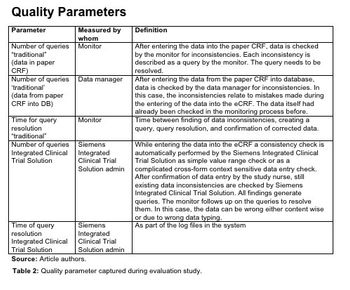
An evaluation of the Munich Pilot and the affects of the EHR-EDC integration solution.







Online Terms & Conditions


Advertisement
Advertisement
Trending on Applied Clinical Trials Online
1
SCOPE Summit 2026 Keynote Panel: Is Radical Acceleration in Clinical Research Possible?
2
SCOPE Summit 2026 Panel Discussion: Diversity in Clinical Trials—What’s Working, What’s Next
3
Accelerate Clinical Trials with AI-Enhanced Financial Management
4
SCOPE Summit 2026 Keynote Fireside Chat: Aligning Purpose, Innovation, and Operational Excellence in Clinical Development
5