
News














Gauging the Risk of Dual Enrollment - Clinical RSVP - Independent Data Integrator






REGULATORY : Avoiding Adverse Event Setbacks in Early Studies TRIAL DESIGN : Proposed Payment Models For the Phase I Volunteer European Pediatric Survey Unveils Research Challenges Also in this issue : FDA Commissioner Wraps Up Her First Year, EU Focuses on Inclusion of Elderly in Trials, Clinical Data Warehouses Stall in Real World, How Pediatric Networks Benefit Children

Adverse events are critical in early phase studies. Early planning can avoid regulatory setbacks.

Industry news focusing on the people and organizations who work in the clinical trials profession.

Updates on cardiovascular drugs in development, including Phase II study results, NDAs, fast-track designations, and related business news.


A look at what the 46th Annual EuroMeeting has in store for attendees.
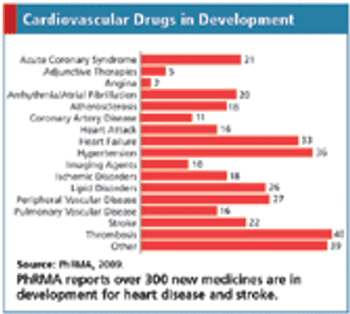
Why experts predict volatile years ahead for the global cardiovascular drug market.

