
News
Advertisement


Advertisement


Formulation Considerations for Over-Encapsulation of Clinical Trial Materials in DBcaps® Capsules - Capsugel Whitepaper

Advertisement












Analytics: The Art and Science of Better - SAS Whitepaper






TRIAL DESIGN : Global Pediatric Research Update Prepare for the Seasonal Diseases That Can Derail Your Trial Also in this issue : EMEA Reorganizes, New FDA Team Eager for 2010, Unnecessary Clinical Data, Cardiac Safety: Consider Centralization

Industry news focusing on the people and organizations who work in the clinical trials profession.
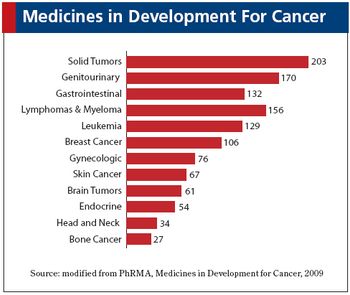
The current oncology drug development market.
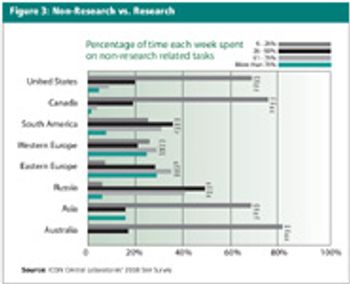
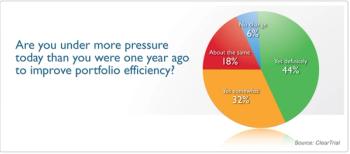
Results reveal insight into the roles, activities, pressures, and priorities of study coordinators.

Cover

Is basing drug efficacy on the site read risky business?
Advertisement
Advertisement
Trending on Applied Clinical Trials Online
1
SCOPE Summit 2026 Keynote Panel: Is Radical Acceleration in Clinical Research Possible?
2
SCOPE Summit 2026 Panel Discussion: Diversity in Clinical Trials—What’s Working, What’s Next
3
Accelerate Clinical Trials with AI-Enhanced Financial Management
4
SCOPE Summit 2026: Reducing Patient Burden Is the Foundation of Wearable Success in Oncology
5