
A brief look back at the recent 18th Annual Partnerships with CROs.



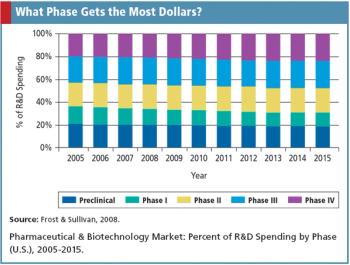
A look at the contract research organization market and its trends toward early phase services.

Industry news focusing on the people and organizations who work in the clinical trials profession.


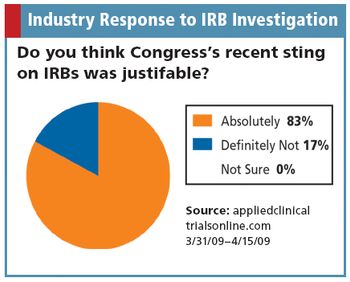
Congressional sting operations put the IRB industry under the spotlight.

The U.S.–EU Transatlantic Administrative Simplication Plan continues on the road of development.



The latest eclinical software in the clinical trials industry.

Industry professionals address critical commentary of the drug development process.

Find out who was honored at this year's Global Conference & Exhibition in Denver.

Enhanced level of QT analysis offers sponsors a more complete foundation to establish drug safety.

Nextrials lauded for its work with industry standards organizations to advance integration of clinical trial data collection platforms with electronic health records.

Phase Forward Signs Multi-Year, Multi-Million Dollar Agreement with Novo Nordisk to Expand Use of InForm EDC Product Across All Clinical Trial Phases.









An interview with Dr. Albert Edwards offers his take on how pilot testing can ease the implementation of eCTD.

