
News





Why ePRO edges out paper as the most reliable data source.

ACT Supplement Cover

Interoperability and Architecture for the Life Sciences Industry

Measuring Recruitment Performance

Removing the Mystique
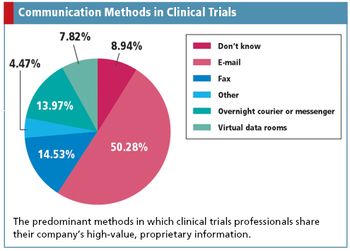
Uncovering the risky communication methods of clinical trials professionals, while discovering a potential Web-based replacement.

London conference underscores foggy climate surrounding clinicaltrials.gov

On the scene with electronic content management.

ACT e-Newsletter Suite

Program-Level Branding

The latest eclinical software in the clinical trials industry.

Industry news focusing on the people and organizations who work in the clinical trials profession.

Subject Recruitment 2009

The Business of Recruitment

Better than a Crystal Ball

DIA Exhibitor Form - PDF Version

How can we streamline late-stage clinical development processes to speed regulatory submissions and approvals?-SAS Whitepaper

MANAGED INNOVATION,ASSURED COMPLIANCE,SAS Whitepaper

MANAGED INNOVATION,ASSURED COMPLIANCE-SAS Whitepaper





