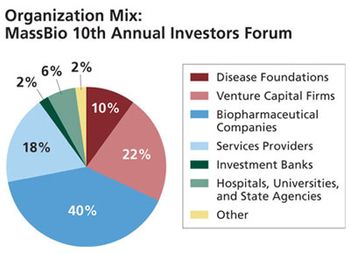
MassBio president Dan McDonald discusses Massachusetts' bio industry and its ability to continually evolve despite the economic downturn.

MassBio president Dan McDonald discusses Massachusetts' bio industry and its ability to continually evolve despite the economic downturn.



Office of Inspector General's report on the FDA's oversight of clinical investigators' financial information.

Cardiocore announced the launch of its redesigned corporate Web site that provides users with a comprehensive view of Cardiocore's history as a top-quality, innovative core lab.

Exco InTouch announces the commencement of its full mobile phone electronic data capture (EDC) study using the Western Ontario and McMaster Osteoarthritis (WOMAC) NRS 3.1 index after the success of their pilot study.

Hans-Georg Eichler, M.D., M.Sc., Senior Medical Officer for the EMEA, has been named the first non-US regulatory liaison to the Clinical Trials Transformation Initiative (CTTI) Executive Committee.

Almac Clinical Technologies announced today that it has added 6,000 square feet of space at its Yardley, Pennsylvania USA headquarters to support the company's continued growth.

Following a complete brand and identity audit, TRAC, has unveiled its completely new look and applied it to everything from company signage and letter heads to a new Web site and exhibition stands.

Hans-Georg Eichler, M.D., M.Sc., Senior Medical Officer for the EMEA, has been named the first non-US regulatory liaison to the Clinical Trials Transformation Initiative (CTTI) Executive Committee.

M2S, a global provider of image management services for biopharmaceutical and medical device clinical trials, announced today that the company has acquired DXA Resource Group (DRG) of Westborough, Mass.

Image Solutions, Inc., a provider of software and services for the life sciences industry, today announced that its Tianjin, China office was granted ISO 27001:2005 certification by the International Organization for Standardization.

NextDocs announced today that they will be joining with Microsoft to deliver a series of live, in-person seminar events across major US cites during the first quarter of 2009.

BASi has announced the awarding of the new European patent for their Culex Automated In Vivo Sampling System.

New for 2009, the Association of Clinical Research Professionals (ACRP), has launched a series of services to expand the online networking and educational resources available to its nearly 20,000 members worldwide.

Pfizer Inc. has agreed to provide the University of Rochester Medical Center (URMC) with a unique set of electrocardiographic data.

PPD, Inc. announced it has entered into a strategic collaboration with Merck & Co., Inc., involving vaccine testing and assay development.

Cardiocore, a centralized cardiac testing lab, announced that it has been selected as a preferred provider by a UK-based specialty pharmaceutical company.
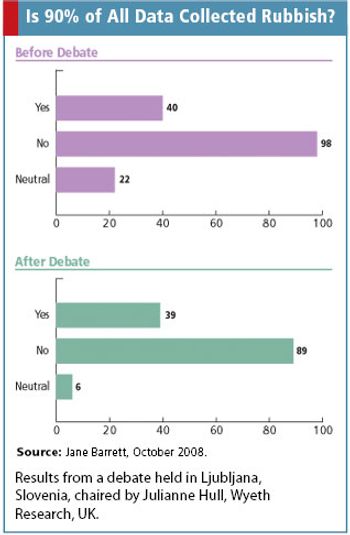
Jane Barrett discusses data quality as she addresses the question raised by DIA's 2nd European Clinical Forum: Is 90% of data rubbish?

The latest eClinical software in the clinical trials industry.

Industry news focusing on the people and organizations who work in the clinical trials profession.

With greater attention and acceptance of biomarker use in clinical trials, the Society of Nuclear Medicine (SNM) is hoping to capitalize on its expertise in this area through the creation of the SNM Clinical Trials Network.

A look into the current state of the eCTD format as well as its future in industry from a global standpoint.

Regional recruitment managers are on the rise, providing a unique approach to onsite subject enrollment.

Good Products, a provider of Enterprise Content Management (ECM) solutions and Clinical Technology Consulting for the pharmaceutical, biotechnology and medical device industries, today announced a major expansion to new UK headquarters in Nottingham, UK.

Entelos, Inc. today announced that it has entered into an agreement with the U.S. Food and Drug Administration (FDA) to use the Entelos Cardiovascular PhysioLab to assess the cardiovascular safety and efficacy of a specific drug class and a set of drugs within that class.

PPD, Inc. and IDA Ireland (Investment and Development Agency) today announced that PPD plans to expand its contract research operations into Athlone, Ireland, by initially establishing a cGMP analytical testing laboratory to meet growing client demand in Europe, Middle East and Africa (EMEA) for these services.

DIA has rescheduled its 3rd Annual Conference on Drug Discovery and Clinical Development in India, which was recently postponed due to recent violence in the area.

Preparing for a busy year ahead, the European Medicines Agency's Management Board adopted the Agency's work program and draft budget for 2009 at its meeting on December 11, 2008.

PPD, Inc. today announced it plans to expand its global central lab services into Singapore in response to growing client demand in Southeast Asia.