
Industry news focusing on the people and organizations who work in the clinical trials profession.

Industry news focusing on the people and organizations who work in the clinical trials profession.
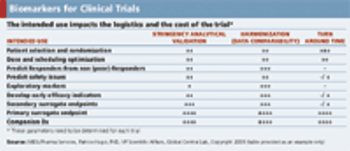
Considering the cost and value of biomarkers when deciding to use them in a clinical trial.
With industry facing technological change, partnering may offer safety solutions.

Learning how such data can provide a real-world view of drug safety and effectiveness.

Regulatory authorities in Italy enforce new legislation that hardens the requirements for education and certification of staff at CROs.


Business Analytics: Six Questions to Ask About Information and Competition-SAS whitepaper




Protecting Sensitive Information in Life Sciences Organizations: Top Three Misconceptions that Put Companies at Risk-Brainloop whitepaper








A case study of the Duke Clinical Research Institute's implementation of Oracle's CTMS shows flexibility is vital.

Janel Firestein, our roundtable moderator, offers her take on the expert discussion and the major challenge she sees to eCTD implementation.



Recent legislative and regulatory changes increase the country's appeal as a destination for research.






