
Metroplex Clinical Research Center (MCRC), a multi-specialty clinical research site in Dallas, has enjoyed a 250 percent increase in business since site executives announced plans to reclaim its assets from Radiant Research in March 2007.

Metroplex Clinical Research Center (MCRC), a multi-specialty clinical research site in Dallas, has enjoyed a 250 percent increase in business since site executives announced plans to reclaim its assets from Radiant Research in March 2007.

TUV SUD has completed the acquisition of all shares of MSOURCE Medical Development to create a new T?V S?D Life Science business unit.

CEO Chairman Jim Walker of Octagon Research Solutions, Inc., a provider of software and services to the life sciences industry, announced today that a new Electronic Data Capture (EDC) Blog entitled, ?EDC Connections? Discussion Forum is now available on the Octagon Research Solutions, Inc. website.

Pharmatech, Inc., a Research Management Organization (RMO), has contracted with five leading pharmaceutical companies to provide research and site management services for their clinical trials using the Just-in-Time (JIT) enrollment strategy.

Velos, Inc., announced today its plans to implement Phase II of its Strategic Plan ? an integration with third party systems and systems-based collaboration across research sites and sponsors.

Abingdon Life Sciences, Inc. is a Drug, Device, Diagnostic Development Management Organization (DDMO), focused on providing drug and device companies with superior services based on an integrated strategic development model.

Pharsight Corporation (Nasdaq:PHST), a provider of software, strategic consulting, and regulatory services for optimizing clinical drug development, and CRI Worldwide, a provider of clinical research services to the global pharmaceutical and biotech industries, announced that they have formed an alliance to offer combined services.

Health Market Science, Inc., a leading source of healthcare provider data and analytics solutions in the United States, announced the launch of Clinical Investigators, their latest innovation for clinical site recruitment.

Praxis, a company specializing in centralized patient recruitment for clinical research studies, was recently awarded Best of Show as well as two Gold International Awards of Excellence (IN-AWE Awards) by the Healthcare Communication & Marketing Association (HCMA)

ProTrials Research, Inc.TM, a leader in the clinical research organization industry, announced the Silicon Valley/San Jose Business Journal has chosen it as one of the area?s Top 50 Women-Owned Businesses for 2008. ProTrials placed seventh on the list that ranks area public and private women-owned companies according to revenue.

ACT's eNewsletter, Lab Views, brings you news specific to the laboratory and its uses in clinical trials including deals, alliances, business developments, people news, and events.

Thermo Fisher Scientific Inc., announced that Veeda Clinical Research has selected its Thermo Scientific Watson LIMS to automate its laboratory processes in its Bioanalytical Research facilies in Ahmedabad, India and Oxford, UK

FDA changes "approvable" and "non-approvable" letters to "complete response" letter at the end of the review period.

The Association of Clinical Research Organizations (ACRO) recently announced the election of Dr. Derek Winstanly as Chair-Elect of the association.

The URMC will collaborate with the FDA to develop a national repository of data that will aid academic and industry researchers studying the electrical activity of the heart.

Almac Clinical Technologies announced today that its Testing Department has completed the ISTQB Software certification.

ClinPhone, a Clinical Technology Organization (CTO) and Cytel Inc., statistical experts in adaptive clinical trials, announced a partnership that unites expertise and clinical technologies

A recently released report details the current status of the clinical trials market in Russia.

Industry news focusing on the people and organizations who work in the clinical trials profession.

Industry news tidbits from around the world.

PharmaPros Takes Electronic Data Lifecycle Management to a New Level with Dataflow Manager.

Online survey allows clinical research professionals to share their experiences with the European Clinical Trials Directive.

The Center for Information and Study on Clinical Research Participation (CISCRP) recognized PharmaNet as this year?s award recipient for ?Supporting the Development of Educational Materials.?

Lawke Links, Cordium Links new joint venture with a Chinese organization, will bring a full range of ECG core lab services to China.
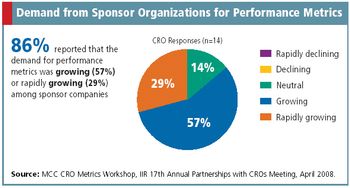
Metrics Champion Consortium standardizes CRO performance metrics across the board.

Symbio LLC, a full service contract research organization, has announced the opening of a new Phase I Clinical Unit in Michigan City, Indiana.

A Russian and Pakistani CRO Announce Network Alliance for Expansion of Global Clinical Trials Services

ICON Medical Imaging Strengthens Cardiovascular Expertise with New Cardiac Scientific Advisory Board Brings together global experts from University of Oxford, Harvard Medical School and University of California

Quanticate Sets Out to Becomes a Major New Player in the Global CRO Market

Leading Massachusetts Clinical Research Companies and Organizations Build Bridges to Life Sciences Centers of Innovation in Europe and Latin America