
Phase Forward Senior Professional Barry Burnstead Honored with Membership Lifetime Award

Phase Forward Senior Professional Barry Burnstead Honored with Membership Lifetime Award

Chief Medical Officer Ed Ikeguchi to Present on UK’s Nationwide EDC Deployment; Senior Director Anne Zielinski to Highlight Data Integration Techniques

Take a pictorial tour of Paris with the editors of Applied Clinical Trials

Program Co-Chairs Iman Barilero, Johnson & Johnson PRD, and Marie Dray, International Regulatory Affairs Group USA, opened the first plenary session with introductions, sharing their vision of what the 18th Annual DIA EuroMeeting is all about: continuing themes throughout the meeting, including pediatrics, advanced therapies, technology platform, pharmacovigilance, risk management planning, and the involvement of patient groups.

Dr. Cynthia Kirk has joined PRA International as Vice President, Global Regulatory Affairs.

The first keynote speaker Georgette Lalis, Head of DG Enterprise and Industry for the European Commission, began her presentation "The Decline of Pharmaceutical Innovation in Europe" by acknowledging, "Innovation is the life blood of the pharmaceutical industry." She added that regulators must be equally innovative, playing a key role in the growth of R&D development in Europe, noting that Europe is facing massive health threats and the health system is under distress.

2,500+ International Professionals from Industry, Academia, and Regulatory Agencies Convene in Paris: 17 Meeting Tracks Including 400+ Speakers, 170+ Exhibits and 14 Tutorials

ClinPhone will be exhibiting its complete range of clinical trial technology services at the 18th Annual DIA Euro Meeting.

Find Out at the DIA Annual EuroMeeting

The Latest Headlines from Europe

Welcome to the Applied Clinical Trials eShow Daily coming from the floor of the DIA 18th Annual EuroMeeting in Paris, France.

Session Highlights for March 6 and 7


09:00-12:30 Graduates Session With Speakers from the DIA and National Health Authorities

Awards broadened, internationalized to showcase solutions in sales and marketing, manufacturing and supply chain, and clinical development.

Creating order from information chaos with the help of the Semantic Web.

Tools for Clinical Trials Professionals

images

Test images

The $18 million acquisition of ClickFind significantly increases customer base and provides the most advanced and extensive eClinical technology platform in the market.


images

Agreement represents a working relationship that will involve joint marketing efforts and a mutual contracting of complementary products and services.

Partnership expands depth and breadth of clinical services
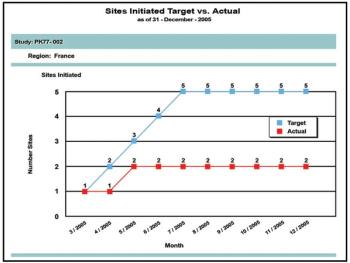
Tools for Clinical Trials Professionals

Authors comment on Kirby et al.'s remedies for placebo response.

United BioSource Corporation (UBC) today announced the acquisition of MetaWorks, Inc., a leading provider of systematic reviews and meta-analyses to inform development, marketing, and regulatory decision-making regarding health care products and services.

Key to improving R&D productivity is the willingness of drug developers to use new discovery tools, such as pharmacogenomics, to accelerate the pace of translating basic research into viable drug candidates, and the aggressive management of clinical trials, through advanced data analysis and outsourcing, to lower cost sites around the world, said Tufts CSDD Director Kenneth I Kaitin.

ArisGlobal, a leading provider of pharmacovigilance software solutions, announces the general availability of SafetyMart, a comprehensive data mining and signal detection application which facilitates in-depth data analysis and proactive benefit/risk assessment of drug safety data.

PRISYM Medica 5 software features RFID capability and ensures compliance with latest FDA regulations