Outsourcing and trial management tool puts professionals in control of all aspects of a study

Outsourcing and trial management tool puts professionals in control of all aspects of a study
Digital writing solution speeds up data harvest while ensuring traceability and capture ease

Virtualization offers companies a practical solution to bypass server limitations.

Dual-purpose package allows submission in both paper and electronic formats

Enhancements to workplace planning tool include "what if" scenario capability

Partnership puts pulmonary data in the hands of researchers when and where they need it

A review of important factors to consider before implementing the solution that will transform EDC.

Web-based dashboard places clinical trial designers squarely in the driver's seat

Tool places sponsors in control of their patient recruitment programs with real-time data reporting
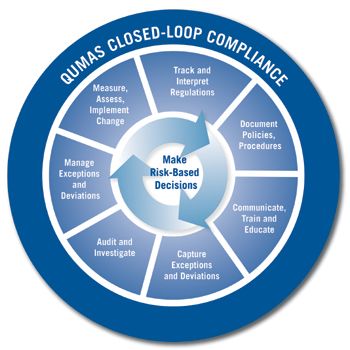
System treats compliance as a single, repeatable process to ensure organizational quality

Web-based system puts sponsors running brain function clinical trials in the know

ACT December 2006 BPA Statement

Washington editor Jill Wechsler provides an updated list of U.S. regulatory agency personnel.

Java applet and Adobe Flash are two of today’s technologies that come close to replicating the dynamic and highly functional environment of desktop software on the Web.

MDS Pharma Ad

Results Demonstrating Encouraging Anti-Tumor Activity and Minimal Toxicity Presented at European Organization for Research and Treatment of Cancer (EORTC)

Submission Instructions

First SACHRP Chairman Resigning

Acquisition Strengthens Constella's U.S. Presence
Software disentangles delivery efficacy from drug efficacy, improving trial results

Silencing a Global Health Challenge that Kills Two Million Children a Year.

ACT would like to welcome Somesh Nigam to our Editorial Advisory Board and introduce him to our readers.

Photo highlights from ExL Pharma's September Conference: Merging eHR & EDC.
Release takes adverse event reporting to a new level, complies with FDA's new rules

Document management suite gets training records tool with eCTD capabilities slated for V4.0

What has the industry and the FDA accomplished this year and what still needs to be done?

From stress levels to job satisfaction, ACT's first salary survey provides an inside look at our industry today.

CEO transition positions Medifacts for growth and expansion

AAIPharma Completes CAC Acquisition