
News tidbits from around the world
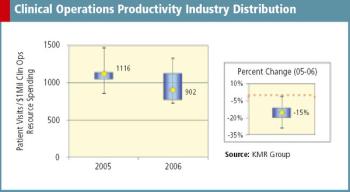
A recent survey from KMR Group shows a 15% decline in clinical operations productivity since 2005.

For the first time n a Phase 1 trial, 3D animation is used for informed consent.

Europe aims to create new regulations regarding hematological malignancies.

A look at the latest industry news

Janet Woodcock is welcomed back as the director of the Center for Drug Evaluation and Research (CDER).

Profect Medical Technologies today announced the introduction of their Clinical Imaging Services division and the hiring of Holly Smith as its Director.

ACT License Agreement

ACT Manuscript Review Form

Leading EDC Company Adds Offerings to Further Drive Efficiencies Across the Clinical Research Process.

UNECA & AU Science with Africa Conference provides a forum for discussion of the development of African health research guidelines and the launch of the AfroGuide Project.

CDISC and HL7 Joint Worldwide Clinical Research Standards Educational Events Planned for 2008

EDC 4.1 System Goes Through Paces; Document Management Announced

Advertising Exhibitors Gallery

New Version Extends Data Cleaning and Query Functions

Clinical Research Information Exchange International One Step Closer to Realizing its Goal of Transforming Clinical Research


Regional centralized labs are emerging to support global clinical trials.


TrialStat's EDC Platform Adds Graphical Reporting Features and More

Recent announcements streamline adverse event reporting

Saul Shiffman, cofounder and chief scientific officer of invivodata, Inc., examines how patient-reported data collection has been transformed since 1987.

Scotland chosen to lead a £26m clinical trial for arthritis sufferers

New Report Offers Hope for Closing Health Gap for African Americans, With Nearly 700 Medicines in Pipeline

Interdisciplinary networking, publications, and conferences are a few of the benefits of society membership.

A list of Web sites focused on the professional and/or potential trial participant.

Organizations offering courses and customized training tools designed to broaden your clinical trials knowledge base.

A comprehensive listing of U.S. departments and offices that includes the telephone numbers of directors, commissioners, and advisors.

These translations help make sense of initials commonly used by clinical researchers around the world.

This newly updated glossary includes even more terms relevant to clinical trials professionals.