
Inside Outsourcing
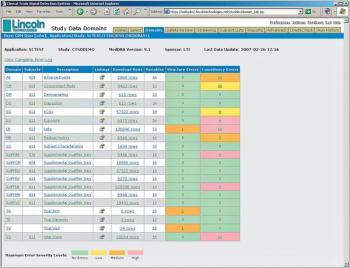
Hosted Service Offering Validates Data Prior to FDA Submission to Improve Review Process

New Version of EDC Solution Builds on Web Capabilities for Integration and Study Deployment

Why strategy is the cornerstone of success: Clearly establishing a registry's strategic purpose can save both time and money, eliminating the collection of extraneous data as well as the added burden it spawns.

Appian International Research Enters Second Year with Ongoing Trials in Seven Countries

SRA International, Inc. to Acquire Constella Group, LLC

OmniComm Systems, Inc. Responds to Industry's Growing Demand for Integrated Electronic Data Capture (EDC) Solutions and Signs 15 New Deals in Second Quarter 2007

Pall Teams Up with RITT to Advance Transplantation Science
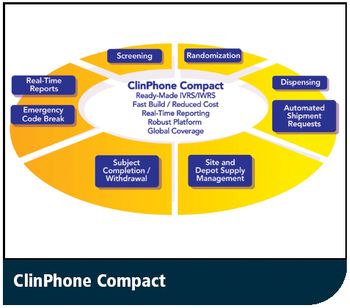
Service Configures Ready-Made Solution for Randomization and Trial Supply Management
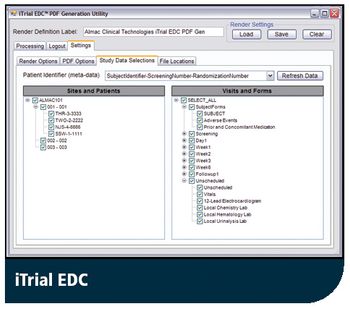
Latest iTrial EDC Includes New PDF Generation Tool to Improve Audit Trail Function

Latest iTrial EDC Includes New PDF Generation Tool to Improve Audit Trail Function

Adaptive Trials Simulation Toolkit Addresses New Ways to Run Clinical Trials

Some starry-eyed vendors can't turn their back on older technology because they're too attached.

LDSE security technology ensures privacy, authenticity, and integrity of 3-D clinical trial image exams.

Groundbreaking new medical journal to offer insight into patient-centered medicine

Selected oncology Web sites to assist readers in becoming more informed about cancer and available clinical trials in this therapeutic area.
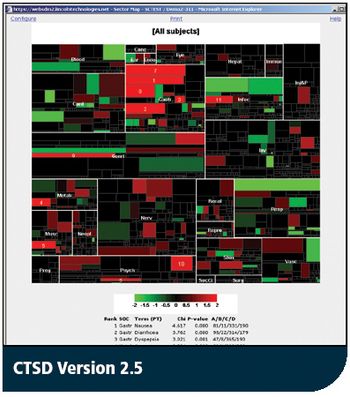
CTSD Version 2.5 provides improved data visualization and enhanced user interface
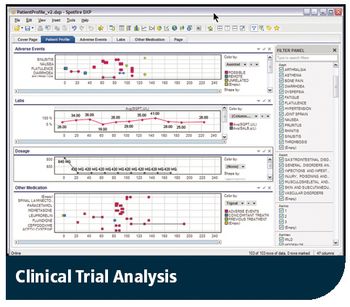
Safety issues pinpointed earlier with Spotfire's interactive analysis data solution

Data JumpStart helps companies deploy standardization and conversion solutions




Regulatory solution suite from ISI migrates to new platform for improved integration
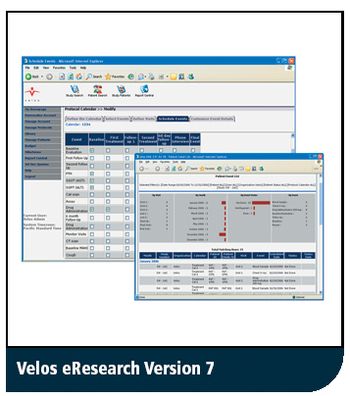
New clinical trial software version provides direct data capture at investigator sites

New data entry interface runs on top of existing software for accelerated navigation

ACT Oncology & Clinical Trials in the 21st Century

ACT July 2007 Ad Performance Study

A number of factors influence the successful electronic collection of patient data, including screen size.

ACT's 15th annual guide highlights EDC, IVRS, and software program development.