
News
Advertisement


Advertisement




Advertisement




Advertisement

Thousands of trials have been halted as a consequence of the coronavirus pandemic, slowing the pace of scientific progress dramatically.





Industry happenings from the last month, all in one place.



Fully-integrated, component-based CDMS offers flexibility, customization, and efficiency.









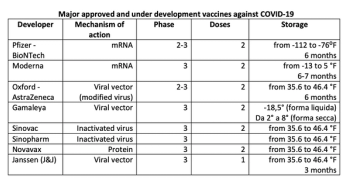
Today's reality and the hopes of tomorrow of the COVID-19 pandemic—an updated mapping of vaccines authorized by regulatory bodies and those close to it.

Advertisement
Advertisement
Trending on Applied Clinical Trials Online
1
SCOPE Summit 2026 Panel Discussion: Diversity in Clinical Trials—What’s Working, What’s Next
2
SCOPE Summit 2026 Keynote Panel: Is Radical Acceleration in Clinical Research Possible?
3
SCOPE Summit 2026: Reducing Patient Burden Is the Foundation of Wearable Success in Oncology
4
SCOPE Summit 2026: Elevating Patient Experience in Clinical Operations
5