
News
Advertisement


Advertisement


The latest industry happenings from the past month, all in one place.

87% of people surveyed said they had no concerns about a decentralized approach to clinical trials.

Advertisement














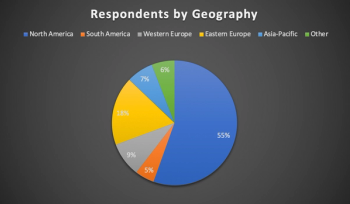
Lessons from six months of conducting clinical trials during the COVID-19 pandemic

The latest happenings of the industry from the past month.







Advertisement
Advertisement
Trending on Applied Clinical Trials Online
1
SCOPE Summit 2026 Keynote Panel: Is Radical Acceleration in Clinical Research Possible?
2
SCOPE Summit 2026 Panel Discussion: Diversity in Clinical Trials—What’s Working, What’s Next
3
Accelerate Clinical Trials with AI-Enhanced Financial Management
4
SCOPE Summit 2026: Reducing Patient Burden Is the Foundation of Wearable Success in Oncology
5