
News









The latest industry happenings from the past month, all in one place.





Findings from Florence Healthcare’s State of Clinical Trial Technology Industry survey about how clinical operations technology changed in 2020 and how they expected it to evolve throughout this year.







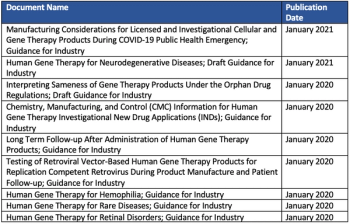
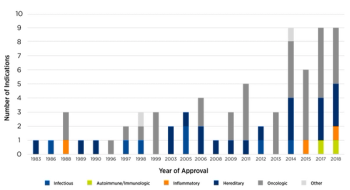
Developing new pathways to overcome study-related challenges is key to realizing the promise of the latest gene therapies.
How partnering with a CRO that has experience in both dermatology and innovative trial approaches can help sponsors differentiate their studies and facilitate recruitment.



The latest people and business news of the industry, all in one place.



New BDO CRO Insights Report reveals high rate of CRA turnover and what organizations are doing to combat it.