
The latest industry happenings of the past month, all in one place.

W2O has announced findings from a research study on consumer attitudes toward data privacy, specifically personal health data.

Recent case study highlights the importance of applying statistical rigor throughout the development and validation processes of biomarkers.

Distinguishing CAR-T and CPI approaches and the clinical trial challenges and complexities associated with each.

A look at one company’s platform approach in enabling the engineering of T-cells to identify and destroy cancer.

Exploring the benefits of capturing and integrating molecular biomarker data within clinical trials to build foundations for data assets.

A compilation of recent notable news developments that pertain to the clinical trials industry.

Perspectives and insights regarding regulatory considerations when planning and conducting immunotherapy studies.

The increasing need for treatments to slow the global spread of COVID-19 has prompted greater cooperation between drug regulatory authorities around the world.

If you are an organization content with patient-centric approaches, you are behind. Today it is about patient-driven drug development.












While there's been hopeful news on treatments and vaccines, sponsors should plan to discuss necessary strategies and contingencies at the outset of new studies or re-opening of halted studies during the COVID-19 pandemic.

A first vaccine against this coronavirus could still take some time to develop, but mRNA vaccine platforms could offer an early breakthrough.
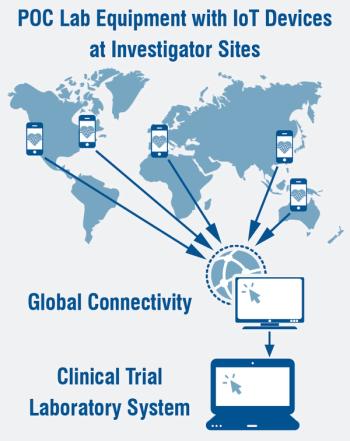
Over the past few years, there has been significant growth in the use of more complex point of care laboratory devices.




An amendment to Pediatric Research Equity Act, as part of the 2017 FDA Reauthorization Act, goes into effect soon aiming to change the landscape and promote pediatric cancer drug development.
