
News

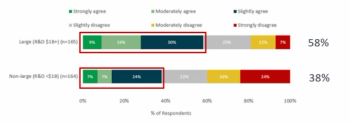
Understanding how your strategy will be re-shaped post-COVID.

The latest industry happenings over the past month, all in one place.


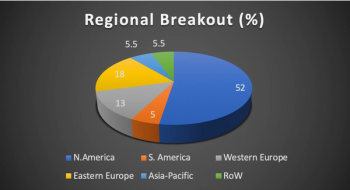
In the third of their survey series, Clinical SCORE uncover the specific hurdles faced by site staff and trial participants during the COVID-19 pandemic and the support needed to overcome them.


To overcome sponsor-related challenges and work to successfully secure more trials, there are tactical steps CROs can take.

While the world focuses on COVID-19, this article provides insight on the current state of the market for antimicrobial resistant (AMR) medicines.
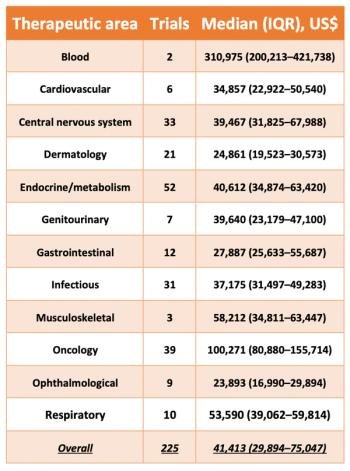
The Institute for Safe Medication Practices has released a new study which breaks down the estimated costs associated with the approval of new pharmaceutical treatments.














While televisits can address many medical concerns, one frequent reservation expressed by healthcare professionals is that patients during these televisits tend to present too narrow a picture of their clinical condition-resulting in non-comprehensive medical assessments.

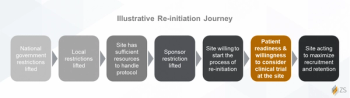
Key takeaways from a survey of 250 oncology patients measuring COVID-19’s impact on patient willingness to participate in clinical trials.


The latest industry happenings, all in one place.
