
News







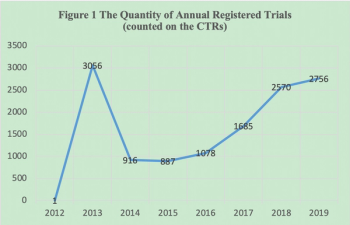
Following the implement of new Drug Registration Regulation in July 2020, the clinical trial registration system will have some major changes in China.


Leveraging approaches in RBQM to enable effective corrective and preventive action processes.

Implementing centralized risk-based monitoring can help meet strict GCP requirements for study conduct, oversight, and recording.

Regulators are urging the use of data monitoring committees to support clinical trial management and decision-making during pandemic.

The latest happenings in the industry, all in one place.




Virtual trials tools are usually selected early in the protocol development stage, however, due to the COVID-19 crisis, regulators are encouraging sponsors to consider integrating virtual elements into clinical trials already in progress.





In order to accelerate clinical R&D and bring drugs to the market quicker, it is imperative that science is complemented by new-age technology such as artificial intelligence and data-driven smart analytics.

Many companies during this time of social distancing have paused trial recruitment completely to focus on currently recruited patients, while others rethink their strategy to better accommodate the new digital-only reality.





