
With technology’s increasing ability to gather and analyze previously unmanageable data sets, and medicine’s forays into genomics and targeted therapies, the time of the master protocol may be at hand.

With technology’s increasing ability to gather and analyze previously unmanageable data sets, and medicine’s forays into genomics and targeted therapies, the time of the master protocol may be at hand.

Amid industry feedback that the growing volume and diversity of eClinical data collected for studies is taxing cycle times, two studies highlight the need to optimize protocol design and executional complexity to overcome these data management burdens.

Sponsors should be aware of the significant implications the Addendum is likely to have on clinical trial planning, conduct, statistical analysis, and interpretation.

Rob DiCicco, VP of Clinical Innovation and Digital Platforms at GSK, will discuss the TransCelerate protocol template initiative in this interview.

Digital innovations are enhancing clinical trials in several ways including recruiting, patient engagement and streamlining data management. Clinical teams will need to develop systematic processes for these new innovations in order to improve their particular trial experience.

Managing a successful Phase III global oncology trial presents one of the most complex challenges drug companies face within the development cycle.

Incorporating original study design accelerates better development decisions.

An insightful review of Remedica's 2005 Clinical Trials: A Practical Guide to Design, Analysis, and Reporting

The protocol should describe the minimization process and processes to minimize systematic bias, as this can influence practical aspects of the study.

FDA plans to rewrite rules governing electronic records while offering new policies to encourage risk-based regulatory approaches to application review and inspections.
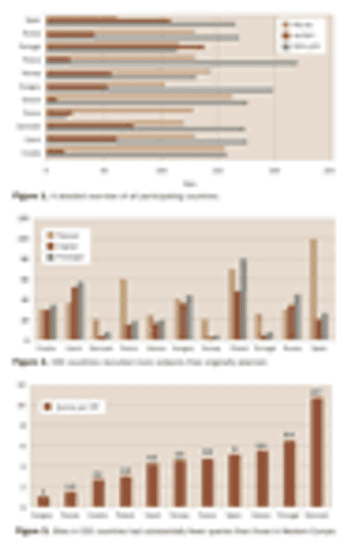
Pharmaceutical companies and contract research organizations are setting up an increasing number of clinical trials in Central and Eastern Europe. The region offers attractive opportunities for sponsors to accelerate subject recruitment and to carry out clinical trials according to harmonized international standards and regulations. Recent concerns about data quality and cost-efficiency at CEE investigational sites, however, have led to regular evaluation of sites' clinical research performance and capacity. Now it is essential to introduce benchmarking and performance measurement to the conduct of clinical trials to identify best practices and to continually improve clinical research.

Pediatrics and geriatrics are the latest hot topics in the EU, so the CPMP is at least starting to help companies develop new clinical trials for children.

The agency is shifting regulation of biotech therapies to its drug center, a streamlining initiative to be implemented by a new FDA commissioner.

Both personal health considerations and altruism helped keep this subject in a long-term study.

The EU?s progress in the battle against cancer measures how effective it is as research supporter instead of regulatory enforcer.

A review of Edyta J. Frackiewicz, Thomas M. Shiovitz, and Stanford S. Jhee's clinical trial book.

A new guideline details basic requirements for submitting a successful application for a proposed orphan drug in the European Union.

José Biller and Julien Bogousslavsky, Eds.

The EU?s Committee for Proprietary Medicinal Products describes the process clinical investigations should follow.

The EMEA updates its positions on placebos, Crohn?s disease trials, and postmarketing surveillance of drugs used by pregnant women.