
Protocol Design
Latest News
Latest Videos

More News

Tufts CSDD study addresses barriers in recruitment.

Data from past clinical trials has provided researchers with a good starting point to create more ECAs.

Pandemic has brought new sense of awareness to respiratory diseases and clinops.

Natural language processing can help simplify protocols of the past, as evidenced by a recent Novartis initiative.

Study uncovers pre-pandemic deviation levels.

Addressing sources of tension on differentiating types of data.

Oncology drug developers must start asking questions in preparation of FDA’s dose optimization initiative.

A look at the prevalence of placebo response in clinical trials and how advanced solutions can mitigate the risk it poses to drug development.

Implementing new strategies with the use of patient-reported outcomes.

Insights from studies on advisory boards and participation burden.

Increased use of remote assets will showcase new endpoints derived from digital health technologies.
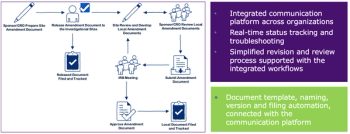
Trials that address multiple questions simultaneously using a master protocol can be operationally complicated. These complexities can be managed, even in studies used to support a marketing application.

87% of people surveyed said they had no concerns about a decentralized approach to clinical trials.

A novel adaptive-block method of randomization to maximize the efficiency of overall treatment group balance, while maintaining balance at investigational centers in smaller sized studies, is proposed.

Recognizing pre-pandemic pain points, such as patient engagement and protocol development, could lead to post-pandemic trial success.

Sponsors are beginning to make their study protocols public as pressure for a COVID-19 vaccine rises.

While not all clinical trial assessments can be done virtually, there are many that can be adapted for virtual use.

This article highlights how C2N Diagnostics and Firma Clinical Research teamed up to create a successful Alzheimer's diagnostic clinical trial while allowing participants to remain in their homes.

Vice President of Global Technology and Product Management, Chris Dailey, and Enterprise Architect at Cenduit LLC, Chris Driver, encourage all sponsors and CROs to take a strategic view of how to make an eClinical ecosystem function at its highest level.

Forming strategic alliances and collaborating to optimize study design can significantly improve pediatric drug development.

With technology’s increasing ability to gather and analyze previously unmanageable data sets, and medicine’s forays into genomics and targeted therapies, the time of the master protocol may be at hand.

Amid industry feedback that the growing volume and diversity of eClinical data collected for studies is taxing cycle times, two studies highlight the need to optimize protocol design and executional complexity to overcome these data management burdens.

Sponsors should be aware of the significant implications the Addendum is likely to have on clinical trial planning, conduct, statistical analysis, and interpretation.

Rob DiCicco, VP of Clinical Innovation and Digital Platforms at GSK, will discuss the TransCelerate protocol template initiative in this interview.

Digital innovations are enhancing clinical trials in several ways including recruiting, patient engagement and streamlining data management. Clinical teams will need to develop systematic processes for these new innovations in order to improve their particular trial experience.













