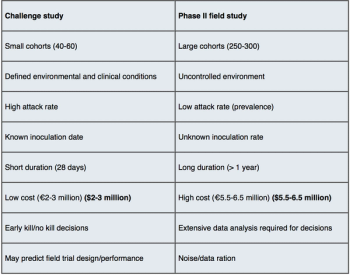
The concept of the human challenge model (HCM) originated from early societal attempts to halt the spread of acute diseases within their own communities

The concept of the human challenge model (HCM) originated from early societal attempts to halt the spread of acute diseases within their own communities



Study shows that industry contributions to R&D go well beyond the applied area of clinical testing.
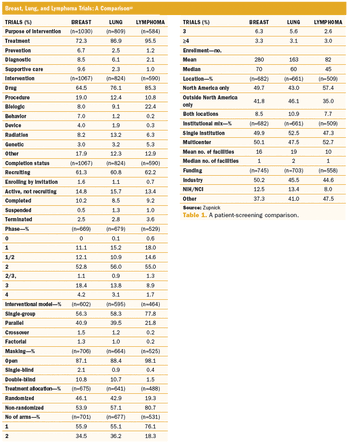
Successful conduct of hematological malignancy trials requires addressing several unique complexities.
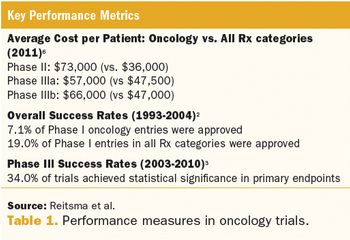
How the practical application of these methods can help overcome the complex demands of cancer trials.

In the healthcare arena, the concept of patient-centeredness has expanded over the last 50 years, beginning as a term to describe patient engagement in self-health management and evolving to include various aspects of patient engagement in healthcare research.1
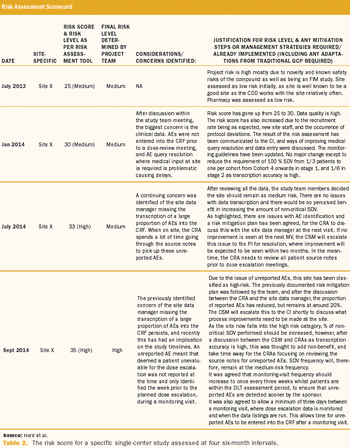
Cancer Research UK (CRUK) followed such advice when it decided to have its Center for Drug Development (CDD) adopt a risk-based monitoring (RBM) approach across its entire portfolio of clinical trials.

Sponsor companies face intense pressure to deliver higher levels of efficiency and drug development performance. A growing number of sponsors are now acting on the belief that improvements in protocol design feasibility hold the key to addressing and easing some of these pressures.
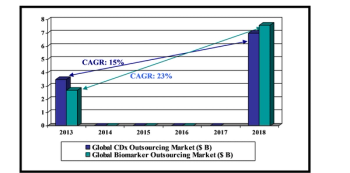
Over recent years global drug companies large or small have recognized that one root cause of the high failure rate in their clinical development lies in the selection of right therapeutic targets.

Case Study, Trigemina TI-001 for high frequency migraine


Research shows that the potential of integrated alliances remains elusive in the near term.

How modeling and simulation clarified complex exposure levels and an orphan drug got approved.

Proving a biosimilar's pharmacokinetic 'equivalence' requires adherence to several unique factors.

A systematic approach for early identification of BP effects during development of new drugs.
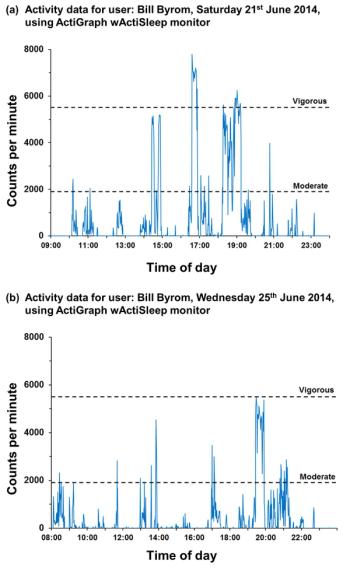
Miniaturization of sensors and circuitry has enabled huge proliferation in the development and commercialization of wearable and external monitoring devices for health and wellness.

Regulatory authorities routinely inspect clinical trials, sites, sponsors, investigators, and other relevant parties to ensure compliance with good clinical practice guidelines.

Conducting Large Simple Trials to answer questions about "real-world" medical interventions is not a recent concept.

Objective internal monitoring can help clinical trial sponsors transform a regulatory mandate into a valuable quality improvement tool.

PCORI underscored existing trends toward increasingly individualized and personalized medical care...

The clinical trials world is starting to adapt computer-aided design.

Companies that don?t collect pharmacokinetic data during Phase II and Phase III clinical trials are missing major dividends.
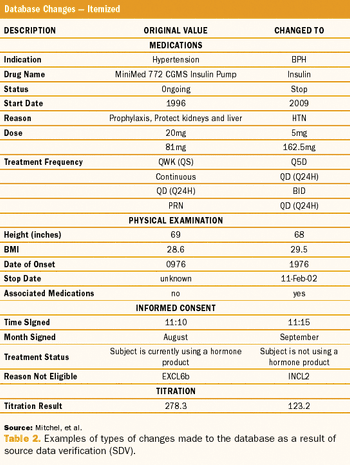
Study results using a quality-by-design method, risk-based monitoring, and real-time direct data entry.

Science exhibit will give children and young adults a rare look inside clinical trial patient experience.