
Tips for reducing site queries and improving workflows.

Tips for reducing site queries and improving workflows.

A look at how real world data and real world evidence are shaping more decentralized trial designs for potential COVID-19 treatments.

Study offers clues for installing rapid R&D tactics post-pandemic.

Rasmus Hogreffe, former Head of Virtual Trials at LEO Innovation Lab, and current VP of Decentralized Trial Innovation at Medable, and Morten Kirkegaard, Head of Clinical Operations and Co-founder at REDO-neurosystems, discuss their experiences with decentralized trials.

A look into the future based off what we've learned about trials during the pandemic.

A look at the future of clinical trial laboratory testing amid the emerging use of new devices at the point of care.

Competency-based and other creative approaches are needed to address the battle for talent in the clinical research workforce.

Dr. Michelle Longmire, Chief Executive Officer and founder of Medable, sits down with Moe Alsumidaie to discuss how the decentralized model will transform how trials are executed for years to come.

Findings from the most recent Biopharma Confidence Index show that the pandemic has substantially influenced biopharma executives’ expectations in key areas like artificial intelligence/machine learning and real-world evidence.

Beyond technology, awareness, and access—cultural competence is key.

A look at how real world data and real world evidence are shaping more decentralized trial designs for potential COVID-19 treatments.

Rasmus Hogreffe, former Head of Virtual Trials at LEO Innovation Lab, and current VP of Decentralized Trial Innovation at Medable, and Morten Kirkegaard, Head of Clinical Operations and Co-founder at REDO-neurosystems, discuss their experiences with decentralized trials.

FDA Commissioner Scott Gottlieb supports approaches that help biomedical research “become more agile and efficient” and reduce the cost of developing therapies, including decentralized trials and how real world data (RWD) and real world evidence (RWE) to support a range of drug development goals.
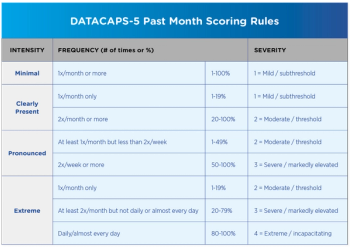
A recurring challenge in assessing and treating Post-Traumatic Stress Disorder or PTSD is the inherently complex, ill-defined, and sometimes downright puzzling nature of its symptoms.

For sponsors who are pursuing the goal of treating Alzheimer’s disease, understanding the obstacles inherent in Alzheimer’s clinical trials can help in planning for, and overcoming, these challenges.

Columnist Moe Alsumidae speaks to key Synteract executives on their acquisition of a dermatology CRO to augment its own expertise and grow the specific services necessary for this therapeutic specialty.

Here are four trends to watch as researchers continue to make headway in the development of biomarkers for cancer immunotherapy.

Until more sophisticated measures are created, a combination of investigator training and new imaging methods is the best way to create accurate, reproducible data across sites.

Due to a shifting landscape because of faster drug approvals, this article offers strategies for sponsors to design clinical trials.

In the search for more effective treatments and cures, there are challenges that sponsors and CROs must confront when conducting IMID clinical trials.

Drug developers are using surrogate endpoints to monitor the effectiveness of immunotherapies, taking into account the differences between immunotherapy and traditional treatments.

While block randomization has been shown to be a more frequently used form of trial randomization design, the use of dynamic randomization has been shown to have optimal treatment group balancing results.

Immunotherapy drugs have led to successes in the oncology space as the list of cancer survivors continues to grow. However, more work must be done to ensure that all patients who can benefit from these life-saving drugs receive them.

Progress and innovation within oncology has accelerated to a paradigm that includes immunotherapy and bio-genomics. Collaborative approaches in the future will continue to transform treatments in hopes of improving patient quality of life.

With significant technology advances in ECG recording through the years, the need to expand on traditional monitoring baselines-and include new variables such as time when designing clinical trial protocols-is important.