When it comes to this overlooked global pandemic, utilizing imaging endpoints in clinical trials is key.

When it comes to this overlooked global pandemic, utilizing imaging endpoints in clinical trials is key.
No longer a futuristic concept, adaptive clinical trials have become a reality that must be considered.

Steady enrollment and optimal trial metrics can become reality with the right processes and tools.

With globalization of trials comes the difficulties of biologic sample logistics. Enter the centralized/decentralized laboratory model.

The use of imaging in trials shows great promise, but proper application requires early dialogue.

IVR systems can capitalize on exposure data and analyze adverse events much earlier.

IVR systems can capitalize on exposure data and analyze adverse events much earlier.

In addition to study timelines and budgets, EDC adoption directly affects the people who run trials.
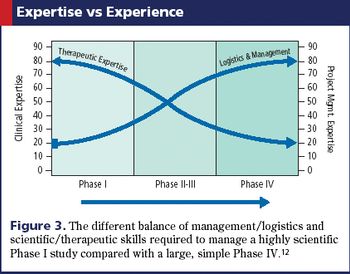
The roles, knowledge, and skill sets of team members also need to adapt with real-time data technology.

How applying statistics to the design, conduct, and analysis of Phase I trials can improve clinical research.
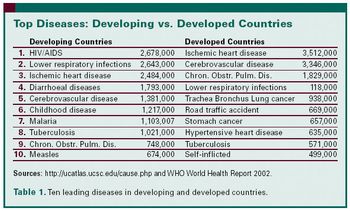
When it comes to R&D all drugs are not created equal, especially oncologics. And an ACT workshop counts the ways.
From Phase I to Phase III, there's a part for personalized medicine to play in improving oncology studies.
A detailed plan is the key to success. But since even the best laid plans can go awry, a back-up plan is necessary.
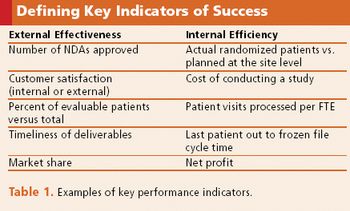
Many factors contribute to the success of a company's metrics group, including high-level support.

It's out with the old, in with the new for clinical development.
A strong data management backbone that includes the use of EDC can make flexible designs an operational reality.

A strong data management backbone that includes the use of EDC can make flexible designs an operational reality.
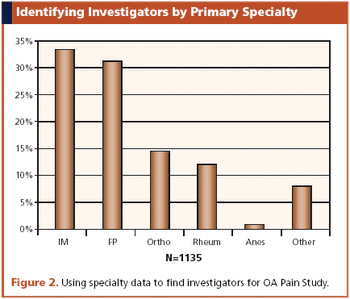
Interactive voice response systems are a good match for adaptive clinical trials and can help keep investigators in the dark.
Increased cost concerns set the stage for health data to play a major role in drug development.
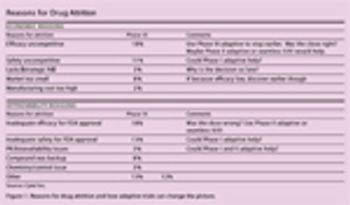
Real examples from recent studies illustrate the risks associated with making such a delicate decision.

An integrated clinical lab EDC system can accelerate decision making and improve subject safety in early trials.
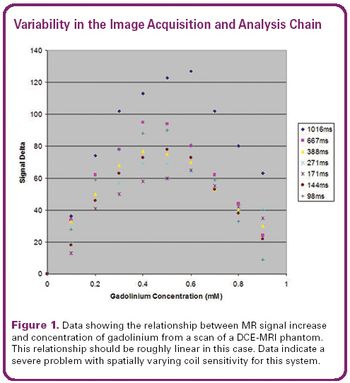
The technique offers a clear window into drug effects and provides strong safety and efficacy evidence.

Open-label extension (OLE) studies are common, but they do not receive as much attention as traditional Phase I through Phase IV studies. Enrollment into an OLE study typically follows enrollment into a randomized, blinded, well-controlled main study. Participants are usually informed at the time they are recruited into the main study that they may elect to enroll in an OLE study after completing the main trial. The stated objective of most OLE studies is to obtain long-term safety and tolerability data.
Electronic solutions such as IVR systems, PDAs, and digital pens exhibit advantages over paper and pencil in PRO data collection.

The need for new trial designs will require flexibility with regard to cost drivers.