
Dave Hanaman, Co-founder of Curavit, discusses his perspectives on decentralized clinical trials.

Dave Hanaman, Co-founder of Curavit, discusses his perspectives on decentralized clinical trials.

Five therapeutic areas that are apt for an all-virtual approach, and that can serve as an entry point for sponsors who are hesitant yet interested in exploring the growing DCT model.

This article sets out a vision of adopting convenient alternative blood sampling approaches that places the needs of the patient at the center and enables active monitoring, before the onset of clinical events, leading to improved healthcare.

Guidance on the unique challenges presented by electronic outcomes.

Using the promising-zone approach can salvage an underpowered trial.

Industry set to make choice on whether COVID-19 trials are the new standard.

Strategies and considerations for adopting decentralized clinical trials.

A look at some of the leading causes of study failure and explain how improving medication adherence could be the key to risk mitigation.

The success of the decentralized model hinges on coordination between stakeholders across the supply chain to ensure that date is secure, medication is delivered on time, and in proper condition.

COVID-19 forces life sciences industry to make long overdue changes.

Pandemic-forced move to remote monitoring calls for industry-wide collaboration.

Upcoming Clinical Trials Day allows industry to look back—and forward.

How asking better questions and leveraging site databases can expand access to more patients.

Analysis of 330,000 clinical trials calls for improved protocol design process.
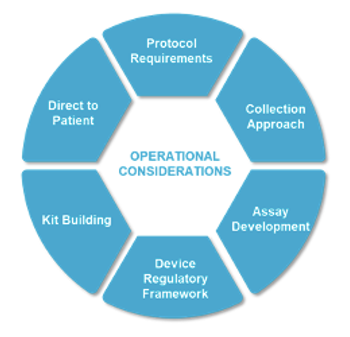
Operational and patient burden considerations for self-collection of blood specimens in clinical trials

A look forward to 2021 for the clinical trials industry.

EVP and General Manager of Hū, April Lewis, discusses why the industry has seen a decline in trial participation and what Hū is doing to combat it.

Regulatory concerns remain despite industry's quick adaptation of decentralized trials.

The industry wide shift to decentralized clinical trials includes considerations for sponsors and CROs such as global availability, global technology, and data integration.

Bayesian methods bring flexibility and speed to clinical trial design and analysis, and with increased access to the necessary computational power, are transforming today’s clinical research.

Both quality personnel and the FDA have predicted some of the issues with pandemic operations, but much is still unknown on the impact these rapidly implemented pandemic processes.

Randomized controlled trials establish strict inclusion and exclusion criteria in an effort to optimize the cohort of trial participants.
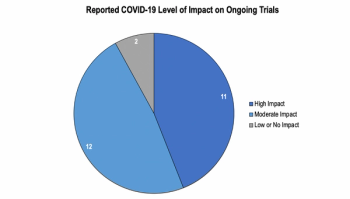
Findings from a Tufts study examining the effects of COVID-19 on clinical trials.

Tips for reducing site queries and improving workflows.

With the assistance of mobile research nursing, in-home visits provide solid alternative to trial participants, especially during COVID-19.