
How Covance has achieved successful outsourcing relationships with pharmaceutical sponsors.

How Covance has achieved successful outsourcing relationships with pharmaceutical sponsors.
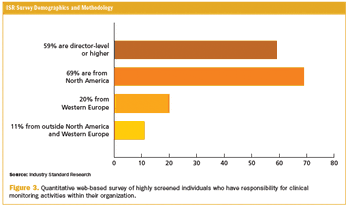
Current commercial forces are working to accelerate the adoption of adaptive monitoring designs.

Survey results show that the recruitment of Latino subjects requires specialized tactics.

Stay in the know with this list of up-to-date, commonly spoken, shortened words that are used often among clinical researchers.

A listing of organizations, colleges, and universities that offer courses specific to advancing the education of clinical research professionals.

Find out the latest on industry organizations and what they offer as membership benefits.

The latest version of the glossary, providing hundreds of definitions for key terminology related to clinical research.

The intent of consent is that participants are comfortable with their choice and can comply with it.

As the popularity of personalized medicine grows the role of the CRO continues to evolve.
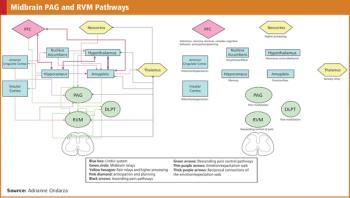
The implications and challenges of the placebo effect on regulatory agency product approval.
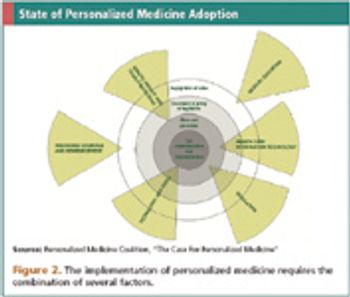
How will the end of one-size-fits-all medicine effect the clinical trial industry?

Use of complementary and alternative medicine among adult cancer patients.

Two industry insiders offer scientific and operational insight into oncology clinical trials.

Information network allows constituencies to share data and knowledge.


Post-approval methods for monitoring the safety of drug exposures in expectant mothers.

Researchers are turning to patient registries to fill rare-disease knowledge gaps.

Stay in the know with this list of up-to-date, commonly spoken, shortened words that are used often among clinical researchers.

A listing of organizations, colleges, and universities that offer courses specific to advancing the education of clinical research professionals.
The latest version of the glossary, providing hundreds of definitions for key terminology related to clinical research.

Find out the latest on industry organizations and what they offer as membership benefits.
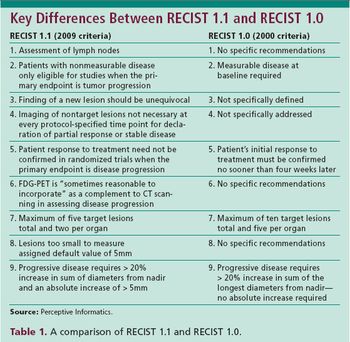
The when and why of using the new RECIST 1.1 criteria without abandoning the old.
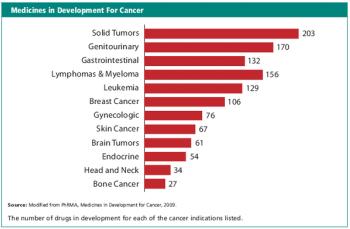
Updates on oncology trials.
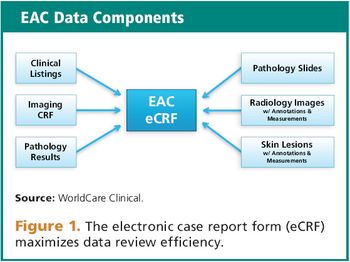
How independent endpoint assessment committees can overcome imaging limitations.

Reductions in unused data will improve study performance, lower costs, and address ethical concerns.