Understanding and using adaptive trial design to achieve the most of its available advantages.

Understanding and using adaptive trial design to achieve the most of its available advantages.
Uses for next generation information management, called health intelligence, in research.
How simulation can help in the planning and implementation of adaptive clinical trials.
Using tumor xenograft technology in drug targets and personalized medicine.
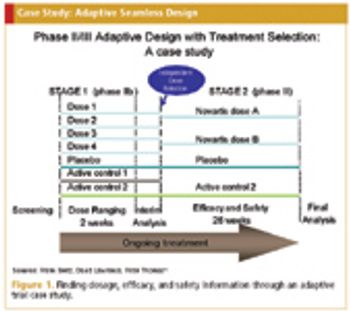
Making adaptations to clinical trials in the early stages of research with the use of interim data.

The spirited decades-old journey of interferon alfa.

A look at the different testing methods and how the results impact drug development.

Developing and utilizing a comprehensive feasibility strategy to avoid risk and ensure the most efficient clinical trial.

Panel members discuss "Working Together to Develop Biomarkers for Safety Monitoring and Surrogate Endpoints," at IIR's Central Labs Partnering conference.

Industry professionals address the changes taking place in pharma and propose an upheaval of current business models to welcome new ones that are adapted for the ever-evolving clinical trials climate.

Realizing the need for flexible technology when implementing EDC in late-phase research.

A clinical trials professional with personal experience as a trial subject discusses the purpose and importance of following protocol.
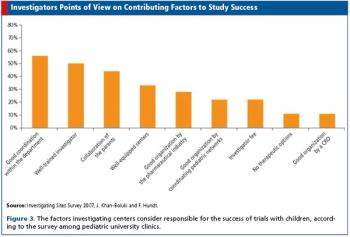
Surveys among pharma, clinics, and investigators shed light on trials in children.


Finding new ways to design and conduct clinical trials in order to prevent Phase III failures.

Acceptance of data ambiguity by the industry is the key to adaptive design adoption and use.
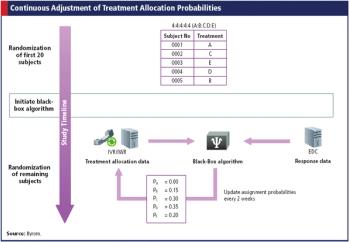
With help from technology, adaptive trials can enhance dose selection and reduce time between phases.

How one workload study measured tasks, time, and resources necessary to run a cancer clinical trial today.

Functional challenges aside, electronic data capture can improve cancer clinical trials.

Current guidelines for reviewing medical images need to be refined and clarified so that consistency becomes the norm.

Dynamic contrast-enhanced MRI meets the needs of emerging cancer therapies that suffocate tumors.
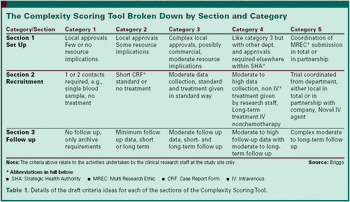
Developing a process and management tool for scoring complexity in cancer clinical trials.

A clear grasp of the methodology behind the design is as important as the software that runs it.

Team and technology benefits are realized at the front-end of the adaptive trial design process.

More complex and demanding protocols are hurting clinical trial performance and success.