
The careful selection of sensor technology prioritized in clinical trials, considering regulatory compliance, data security, device characteristics, and the specific needs of the trials for successful and confident implementation.

The careful selection of sensor technology prioritized in clinical trials, considering regulatory compliance, data security, device characteristics, and the specific needs of the trials for successful and confident implementation.

There is a growing need to refocus efforts on how diversity is achieved in clinical trials, because some are doing it wrong despite their best intentions.

In the ever-evolving landscape of healthcare, it's vital for organizations to take steps toward bridging the gap between their clinical and marketing teams.

Webinar Date/Time: Thursday, November 30th, 2023 at 3pm EST | 12pm PST | 8pm GMT | 9pm CET

Webinar Date/Time: Tue, Nov 14, 2023 11:00 AM EST

Webinar Date/Time: Thursday, November 2nd, 2023 at 10am EDT | 7am PDT | 2pm GMT | 3pm CET

Webinar Date/Time: Wednesday, November 8th, 2023 at 11am EST | 8am PST | 4pm GMT | 5pm CET

Experts weigh in on the latest advances and adoption trends.

Approaching protocol design and operational planning with patient empathy.

Webinar Date/Time: Wed, Sep 20, 2023 10:00 AM EDT


In this Q&A, Matt Smith, Vice President Research Site Development, Slope discusses the competition pharma companies and trial sponsors face when trying to become a sponsor of choice and build trust with sites and patients.

Perceived barriers of DCTs can be quickly overcome to ensure their viability in clinical research.
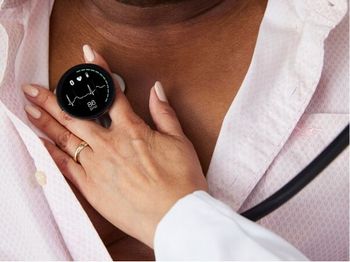
Eko Health developed the device, which has received 501(k) clearance from the FDA.

Webinar Date/Time: Thursday, June 22nd, 2023 at 9am EDT | 6pm PDT | 2pm BST | 3pm CEST

‘No longer just a cool technology,’ efforts to validate these signals are evolving.

Increased data output from the use of devices can accelerate trials for this therapeutic area in need of new therapies.


Presentation at SCOPE 2023 highlights tools such as interactive schedules and benchmarked data which can increase patient centricity.

Webinar Date/Time: Tue, May 9, 2023 11:00 AM EDT

Webinar Date/Time: Option 1: Thursday, April 13th, 2023 at 9am EDT | 2pm BST | 3pm CEST Option 2: Thursday, April 13th, 2023 at 2pm EDT | 11am PDT | 7pm BST

Webinar Date/Time: Thu, May 4, 2023 10:00 AM EDT

John R. Sims, senior vice president and head of global development, and Dawn A. Brooks, director of clinical development; both with Eli Lilly discuss the design of the TRAILBLAZER-ALZ program.

Research can reveal vital information about treatments in the long run.

Survey examines trial enrollment motivation among diverse populations.